
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:EtO.
OEt
H3C
OEt
1. NaOEt, EtOH
2. H₂O*
EtO
EtOH
OEt
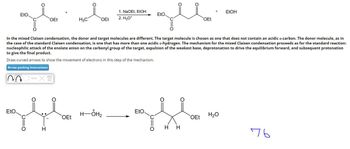
In the mixed Claisen condensation, the donor and target molecules are different. The target molecule is chosen as one that does not contain an acidic α-carbon. The donor molecule, as in
the case of the standard Claisen condensation, is one that has more than one acidic a-hydrogen. The mechanism for the mixed Claisen condensation proceeds as for the standard reaction:
nucleophilic attack of the enolate anion on the carbonyl group of the target, expulsion of the weakest base, deprotonation to drive the equilibrium forward, and subsequent protonation
to give the final product.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
EtO
CX
ཚོད་ཡིན་རྒྱུ་ཡིག་དཔྱ་ རྩཔའི་བད་ཨ—- བམེད་ཚིག་དྲིས་ ༣༠
+
H-OH2
OEt
H
HH
OEt
H₂O
76
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank the relative order of elution of the following sets of compounds, from the fastest eluting compound to the slowest, and explain your assignment. NH2 OH NH2 NH2 4 1 2.arrow_forwardShow the organic chemical reaction mechanism of the synthesis of 1-bromobutane from 1-butanolarrow_forwardWrite mechanisms for thesearrow_forward
- Use the Le Châtelier's Principal with a conversion rate of 30% A→B and 45% B→A. After reaching equilibrium, adjust the quantities to A=10 and B=90. How many additional time iterations (rows) are required before equilibrium is re-established (i.e. when the number you type on the left first equals the number you type on the right)?arrow_forwardFor the given SN2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group. H -NH₂ H Organic product + Inorganic product H organic product inorganic product Select Draw Rings More Erase Select Draw Rings More Erase с / с H N 1 Q2 Q Select the statement that properly identifies the nucleophile, substrate, and leaving group. CH₂I is the substrate, NH₂ is the nucleophile, and I is the leaving group. O NH₂ is the substrate, CH₂I is the nucleophile, and I¯ is the leaving group. Or is the substrate, NH₂ is the nucleophile, and CH₂I is the leaving group. G H N I Q2 Qarrow_forwardWrite a mechanism for this reaction below.arrow_forward
- Chemistry CH3 o-nitrotoluene CH3 NH CI NO2 NH,HCO, 10% Pd/C Step 1 1) NaOAc, H₂O ayla CI CH3 o-toluidine NH3*,CI CH3 CI 4 2-chloro-N-o-tolilpropanami 2) Step 2 2 o-toluidine assignment I need complete mechanism IZarrow_forward1a. What is the mechanism for reaction 2? 1b. What is the mechanism for reaction 3? a Electrophilic addition b Electrophilic substitution c Radical substitution d Rearrangement 2. What reagents are needed in reaction 3? a 3H2, Rh (with high pressure) b H2, PtO2 c KMnO4, H+ d Br2, uvarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY