
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
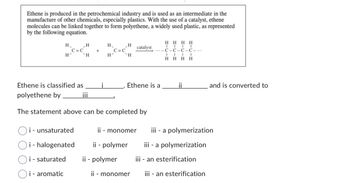
Transcribed Image Text:Ethene is produced in the petrochemical industry and is used as an intermediate in the
manufacture of other chemicals, especially plastics. With the use of a catalyst, ethene
molecules can be linked together to form polyethene, a widely used plastic, as represented
by the following equation.
H
H
H
`H
C=C
Ethene is classified as
polyethene by
i - unsaturated
i - halogenated
i - saturated
Oi- aromatic
+
i
H₁
H²
C=C
H
H
catalyst
Ethene is a
The statement above can be completed by
HHHH
C-C-C-C-...
I
HH
II
HH
ii
and is converted to
ii - monomer iii - a polymerization
ii - polymer
iii - a polymerization
ii - polymer
iii - an esterification
ii - monomer iii - an esterification
Expert Solution
arrow_forward
Step 1
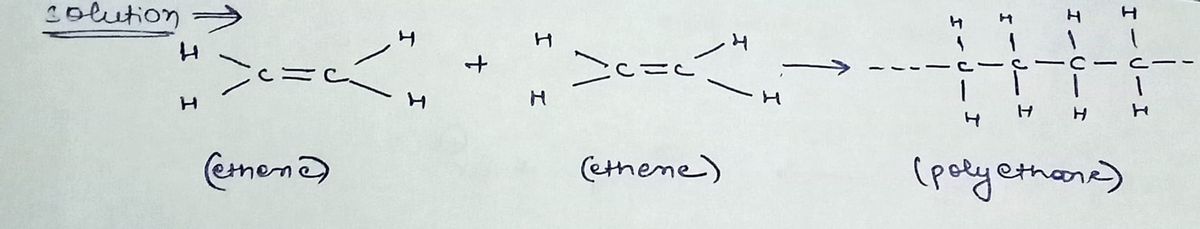
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 12. A way to produce a highly cross-linked polyester is to use glycerol. Alkyd resins are a polymer of this type. The polymer forms tough coatings when baked onto a surface and is in used in automobile paints and large appliances. Draw the structure of the polymer formed from the condensation of one glycerol and three phthalic acid molecules: H₂C-CH-CH₂ COH OH OH OH + сон Glycerol phthalic acidarrow_forwardPlease don't provide handwritten solutionarrow_forwardWrite or introduce the following topics of polymer : • Thermoplastic/thermoset elastomer •Addition/condensation • Step/chain polymerization • How chains interact -> strength and consequences of crosslinking • Molecular average vs number average -> polydispersityarrow_forward
- Charles Goodyear discovered that if sulfur is added to rubber and the mixture is heated, the rubber hardens, becomes more resilient, and does not melt. This process is referred to as and involves the formation of sulfur bridges between the methyl side groups on different chains. A polymerization sintering c hybridization D linking E) vulcanizationarrow_forwardDraw the structure(s) of the monomer(s) used to make each of the following polymers Define whether each polymer is a A. homopolymer or B. copolymer Define whether each polymer can be prepared by C. free radical polymerization or D. condensation polymerizationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY