
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
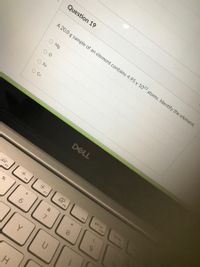
Transcribed Image Text:Question 19
A 20.0 g sample of an element contains 4.95 x 1023 atoms. Identify the element.
O Mg
O Fe
O Cr
DELL
prt sc
F10
home
FI1
F5
F6
F7
F8
F9
8.
* CO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Antimony has two naturally occuring isotopes, 12 Sb and 123Sb. 12'Sb has an atomic mass of 120.9038 u, and 123Sb has an atomic mass of 122.9042 u. Antimony has an average atomic mass of 121.7601 u. What is the percent natural abundance of each isotope? 121 Sb: % 123 Sb:arrow_forwardWhich sample contains the greatest number of atoms? (No calculations necessary). Group of answer choices 68 g 31Ga 70 g 34Se 34 g 15P 190 g 79Auarrow_forward6. What is the mass of 6.20 x 1025 atoms of sulfur?arrow_forward
- A 12.6712.67 g sample of NaBr contains 22.3422.34% Na by mass. Considering the law of constant composition (definite proportions), how many grams of sodium does a 6.636.63 g sample of sodium bromide contain?arrow_forwardThe average atomic mass of lithium is 6.925 amu. Lithium has two natural isotopes. The larger one has a 92.5% abundance and is 1 u larger than the smaller isotope. Dtermine the isotopes.arrow_forwardSymbol Abundance in Nature Protons: Periodic Table Neutrons: Electrons: H. Не Li Be C Ne My Isotope Symbol Carbon-13 Abundance in Nature +1 Stable O Mass Number 13 Neutrons Atomic Mass (amu)arrow_forward
- A sample of Co weighs 77.2 grams. Will a sample of Sn that contains the same number of atoms weigh more or less than 77.2 grams? OA sample of Sn weighs less than 77.2 grams. OA sample of Sn weighs more than 77.2 grams. Calculate the mass of a sample of Sn that contains the same number of atoms. 9 Sn Mass =arrow_forwardBronze medals are made from an alloy composed of 93.8% copper, 5.1% tin, and 1.1% zinc (measured by mass). What percentage of the atoms in this alloy are tin atoms?arrow_forwardNa + 2 H2O à 2 NaOH + H2 If 50 g of sodium (Na) is placed in water, what mass of hydrogen gas (H2) is produced?arrow_forward
- One atom of calcium weighs 6.02 x 1023 amu 20 g 20 amu 40.08 g none of thesearrow_forwardIf 5.216 g of a pure element contains 2.579x10^22 atoms what is the element?arrow_forwardDid you ever want to know what you're made of? The table below shows the elemental break down of the typical human body. How many oxygen atoms are present in a typical 68.0 kg chemistry teacher? Percent by Percent by Element mass Element mass Охудen 65.0 Potassium 0.4 Carbon 18.5 Sulfur 0.3 Hydrogen 9.5 Sodium 0.2 Nitrogen 3.2 Chlorine 0.2 Calcium 1.5 Magnesium 0.1 Phosphorus 1.0 Other 0.1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY