
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
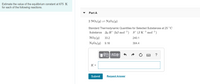
Transcribed Image Text:Estimate the value of the equilibrium constant at 675 K
for each of the following reactions.
Part A
2 NO2(9) = N2O4(g)
Standard Thermodynamic Quantities for Selected Substances at 25°C
Substance Af H° (kJ mol 1)
S° (J K¯1 mol-1)
NO2(9)
33.2
240.1
N204(g) 9.16
304.4
ΑΣφ
?
K =
Submit
Request Answer
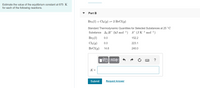
Transcribed Image Text:Estimate the value of the equilibrium constant at 675 K
for each of the following reactions.
Part B
Br2 (1) + Cl2 (g) = 2 BrCl(g)
Standard Thermodynamic Quantities for Selected Substances at 25 °C
Substance Af H° (kJ mol¬1) S° (JK-1 mol1)
Br2 (1)
0.0
152.2
Cl2 (9)
0.0
223.1
BrCl(g)
14.6
240.0
ΑΣΦ
K =
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Be sure to answer all parts. Calculate K at 298 K for the following reaction: SrSO4(s) Sr²+ (aq) + SO² (aq) x 10 (Enter your answer in scientific notation.)arrow_forward2) Calculate the numeric value of the equilibrium constant K for reaction at 298 K: 2 NO2 (g) → N₂O4 (g) from Data Section: les of das Athina.pdf-Adebe Acrobat Reader (54-b0) You SignWindow H Tools Home 827-31.40 - Tables of data Atkin x a Table 2.7 (Continued) Neon Ne(g) Nitrogen N₂(g) N(g) NO(g) N₂O(g) NO₂(g) N₂O₂(g) N₂O5(s) N₂O,(g) 1081 (12 of 4) TTATO (11 A M/(g mol-¹) 20.18 28.013 14.007 30.01 44.01 46.01 92.1 108.01 108.01 OO 201 8. P AH*/(kJ mol-¹) 0 0 +472.70 +90.25 +82.05 +33.18 +9.16 -43.1 +11.3 174 17 AG/(kJ mol-¹) 0 0 +455.56 +86.55 +104.20 +51.31 +97.89 +113.9 +115.1 ℗ 30 31 x Sign In &00 S B D 2 C %arrow_forwardFor the reaction X(g) + 2Y(g) = 3Z(g) K, = 2.08x10-2 at a temperature of 325 °C. Calculate the value of Kc . Express your answer numerically. View Available Hint(s) V ΑΣφ K = %3Darrow_forward
- Please don't provide handwritten solution .....arrow_forwardIs the following chemical reaction spontaneous or non-spontaneous? 31 (aq) + H3ASO4(ag) + 2H* 3 (aq) + H3ASO3(aq) + H2O) (aq) Substance or ion AH°{(kJ/mole) S° (J/mole*K) -55.19 180.7 I (aq) H3ASO4(aq) -345.69 212.34 -11.23 11.09 H* (ag) 69.77 -89.6 13 (aq) H3ASO3(aq) -301.27 199.78 H20 (1) -285.8 69.95 the enthalpy must be calculated first both spontaneous and non-spontaneousarrow_forward< ✓ 11 Question 25 of 25 (1 point) | Question Attempt: 1 of 2 ✓ 12 ✓ 13 ✓ 14 Here are some facts about the reaction: ✓ 15 At -5.67 °C the concentration equilibrium constant K = 7.0 for a certain reaction. с • If the reaction is run at constant pressure, 101. kJ/mol of heat are absorbed. X 16 -1 1 • The constant pressure molar heat capacity C₂ = 2.23 J-mol K Р Using these facts, can you calculate Kat 9.4 °C? • If the reaction is run at constant pressure, the volume increases by 13.%. If you said yes, then enter your answer at right. Round it to 2 significant digits. If you said no, can you at least decide whether Kat 9.4 °C will be bigger or smaller than Kat -5.67 °C? O Yes. O No. 0 17 Yes, and K will be bigger. No. Yes, and K will be smaller.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY