
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
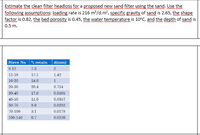
Transcribed Image Text:Estimate the clean filter headloss for a proposed new sand filter using the sand. Use the
w wm w
www w
following assumptions: loading rate is 216 m/d.m?, specific gravity of sand is 2.65, the shape
factor is 0.82, the bed porosity is 0.45, the water temperature is 10°C, and the depth of sand is
0.5 m.
www
www.w wtw
ww w w w
w www
d(mm)
Sieve No
% retain
8-12
7.3
12-16
17.1
1.42
16-20
14.6
20-30
20.4
0.714
30-40
17.6
0.0505
40-50
11.9
0.0357
50-70
5.9
0.0252
70-100
3.1
0.0178
100-140
0.7
0.0126
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- What are the four elements of corrosion cell ? Give brief description for each of the element.arrow_forwardA 4-m high and 6-m-wide wall consists of long 15-cm x 20 cm cross section horizontal bricks (k of 0.69 W/m K) separated by 2-cm thick plaster layers (k of 0.2 W/m K). There are also 2-cm-thck plaster layers on each side of the brick and a 3-cm-thick rigid foam (k of 0.025 W/m K) on the inner side of the wall. See figure below. The indoor and the outdoor air temperatures are 18 and -8oC, respectively. Convective heat transfer coefficient for inside and outer surfaces are 8 and 22 W/m2 K, respectively. Assume 1-D heat transfer. Neglect radiation. assume any plane wall normal to the x-direction is isothermal, draw the thermal circuit and calculate the heat transfer rate through the wallarrow_forwardRepresents and describes by means of a diagram the main parts of the evaporation equipment.arrow_forward
- 2. A 3-D printer was used to fabricate a porous cartilage scaffold using a hydrogel solution. The hydrogel (viscosity was 3 x 10 Pa-s) was added to the extruder, and a pressure of 5 bar was applied to extrude 500 mL of the solution over 10 minutes to form the desired shape. The needle attached to the extruder had a length of 50 mm and an inner diameter of 1.0 mm. Determine the pressure drop needed to complete this fabrication process.arrow_forward1. During a carburization process for the surface treatment of steel, how much processing time in minutes is needed to increase the carbon content at 0.4% up to 0.85% at (a) 0.5 mm and (b) 1 mm below the surface? Assume carbon content at the surface is 0.95 % and steel inside has background carbon of 0.1% and diffusivity is 1.1 x 10-13 m²/s.arrow_forward21. A plate and frame filter press is used to filter a compressible sludge (S = 0.45) at 50 psia for 2 hours. Washing is done at 30 psi with wash water equal to 10% of the filtrate volume collected. The washing time is a. 100 min b. 127 min c. 85 min d. 205 minarrow_forward
- Help me, pleasearrow_forwardExplain in detail about hydration and setting times of cementarrow_forwardLayers %R AT Surface Ts 1. Inside air film 20 0.037 2. hardboard, medium density 25mm 0.073 3. concrete blocks, rectangular core:lightweight aggregate 2 core 200mm 0.118 4. expanded poystyrene molded beads 100mm 0.764 5. Outside air film in vented cavity 0.009 What is the AT of the above shown wall for layer 4 (expanded polystyrene molded beads 100mm)? The inside air temperature is +20°C, and the outside air temperature is -4°C. The units for your answer are °C, and your answer is correct if it is within 10% of the correct answer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The