
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
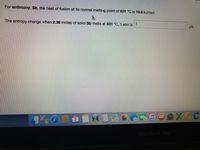
Transcribed Image Text:For antimony, Sb, the heat of fusion at its normal melting point of 631 °C is 19.6 kJ/mol.
The entropy change when 2.36 moles of solid Sb melts at 631 °C, 1 atm is
1
J/K.
FER
7
MacBook Pro
Expert Solution
arrow_forward
Step 1
Question is based on the concept of chemical thermodynamics.
enthalpy of fusion is defined as the amount of heat required to change one mole of solid substance into liquid. entropy of fusion can be calculated by dividing enthalpy with melting point and multiplying with moles of the substance.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the total energy (kJ) required to heat a 17.5 g sample of benzene (C6H6) from 0 °C to 97.2 °C molar mass: 78.11 g/mol cs,solid = 1.51 J/g*°C cs,liquid = 1.70 J/g*°C cs,gas = 1.05 J/g*°C ΔHfus = 9.9 kJ/mol ΔHvap = 33.8 kJ/mol Tf = 5.6°C Tb = 80.08°Carrow_forwardA certain liquid has a vapor pressure of 92.0 Torr at 23.0 °C and 259.0 Torr at 45.0 °C. Calculate the value of AHap for this liquid. AH vap = Calculate tl x10 boiling point: TOOLS s liquid. kJ/mol °Carrow_forwardHow much energy is required to vaporize 41.4 g of dichloromethane (CH2Cl2) at its boiling point, if its ΔHvap is 31.6 kJ/mol?arrow_forward
- A certain substance has an enthalpy of fusion of 6.12 kJ/mol and an entropy of fusion of 22.3 J/mol/K. Calculate the approximate melting point of the substance. Leave the answer in Kelvin.arrow_forwardOxygen (O₂) is necessary for cellular respiration; it must be transported from the lungs to tissues through the bloodstream. However, O₂ is not very soluble in water. The Keq of oxygen dissolving at 300.0 K is 7.64 x 10⁻⁴ and the Keq at 320.0 K is 5.23 x 10⁻⁴. Calculate the ∆H(dissolve) for oxygen solubility in water, in kJ/mol.arrow_forwardFor silicon, Si, the heat of fusion at its normal melting point of 1410 °C is 46.4 kJ/mol. The entropy change when 2.12 moles of liquid Si freezes at 1410 °C, 1 atm is J/K.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY