
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![**Title: Calculating the Equilibrium Constant \( K_p \) for a Reaction**
---
**Instructions:**
Write the expression for the equilibrium constant \( K_p \) for the following reaction.
- Enclose pressures in parentheses and do not write the chemical formula as a subscript. For example, enter \( (P_{\text{NH}_3})^2 \) instead of \( P_{\text{NH}_3}^2 \).
- If either the numerator or denominator is 1, please enter 1.
**Chemical Reaction:**
\[ 3 \text{PbO}_2 (s) \rightarrow \text{Pb}_3\text{O}_4 (s) + \text{O}_2 (g) \]
**Input Box:**
There is an input box provided to enter the expression for \( K_p \).
**Symbols Available:**
- Common Symbols: \( x^2, x^3, x_1, x_2 \)
- Greek (Upper): Alpha, Beta, Gamma, etc.
- Greek (Lower): alpha, beta, gamma, etc.
- Arrows: \(\rightarrow, \leftrightarrow\)
- Other: \( (s), (l), (g), (aq) \)
**Options:**
- Submit Answer
- Retry Entire Group
- 9 more group attempts remaining
---
**Guide for Students:**
1. Identify the gaseous components in the reaction as solids and liquids are not included in the expression for \( K_p \).
2. Use the provided symbols to correctly format your answer.
3. Check if the reaction involves only solids, if so, the equilibrium constant, \( K_p \), would be determined by gases present.
In this reaction, since \( \text{O}_2 (g) \) is the only gaseous product, your expression for \( K_p \) will involve only this component.](https://content.bartleby.com/qna-images/question/3e5f0bee-bbde-421f-8347-e8b63f453811/b78673ee-5d24-4ba9-b937-78c1404a9cd2/3bbm90o_thumbnail.jpeg)
Transcribed Image Text:**Title: Calculating the Equilibrium Constant \( K_p \) for a Reaction**
---
**Instructions:**
Write the expression for the equilibrium constant \( K_p \) for the following reaction.
- Enclose pressures in parentheses and do not write the chemical formula as a subscript. For example, enter \( (P_{\text{NH}_3})^2 \) instead of \( P_{\text{NH}_3}^2 \).
- If either the numerator or denominator is 1, please enter 1.
**Chemical Reaction:**
\[ 3 \text{PbO}_2 (s) \rightarrow \text{Pb}_3\text{O}_4 (s) + \text{O}_2 (g) \]
**Input Box:**
There is an input box provided to enter the expression for \( K_p \).
**Symbols Available:**
- Common Symbols: \( x^2, x^3, x_1, x_2 \)
- Greek (Upper): Alpha, Beta, Gamma, etc.
- Greek (Lower): alpha, beta, gamma, etc.
- Arrows: \(\rightarrow, \leftrightarrow\)
- Other: \( (s), (l), (g), (aq) \)
**Options:**
- Submit Answer
- Retry Entire Group
- 9 more group attempts remaining
---
**Guide for Students:**
1. Identify the gaseous components in the reaction as solids and liquids are not included in the expression for \( K_p \).
2. Use the provided symbols to correctly format your answer.
3. Check if the reaction involves only solids, if so, the equilibrium constant, \( K_p \), would be determined by gases present.
In this reaction, since \( \text{O}_2 (g) \) is the only gaseous product, your expression for \( K_p \) will involve only this component.
Expert Solution
arrow_forward
Step 1
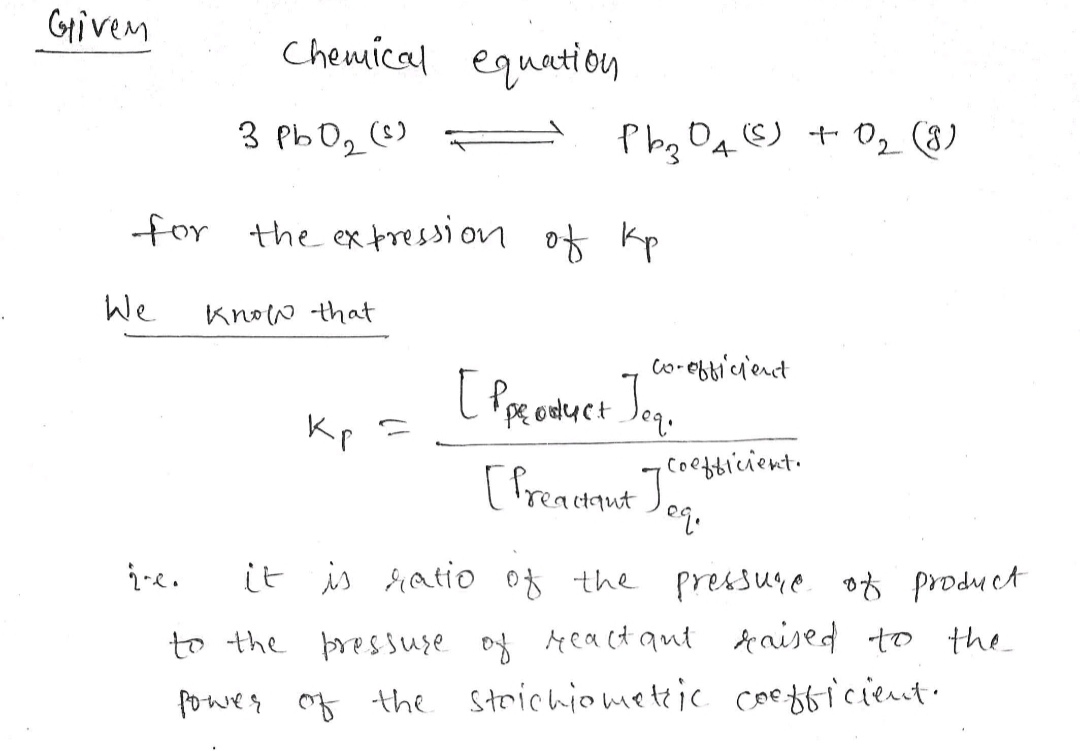
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the equilibrium reaction and its equilibrium constant expression. [ICI]? 1(g) + Cl,(g) 2 2ICI(g) K = L] C] For the reaction 2ICI(g) = 1(g) + Cl,(g) select the equilibrium constant expression. K' О к [ICI]4 2|ICÍ] [L] [CL] L][Cl] [ICI]? [ICi]* K': K' = [Ici]? K' %3D L][CL]arrow_forwardFor the reaction N,0,(g) = 2NO,(g) Kc = 4.66 × 10-3 at 25°C . 2.50 g N,04 and 0.190 g NO, are introduced into a 2.00-L reaction vessel. After equilibrium is achieved, what is the concentration of NO,?arrow_forwardA chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 NO(g) + Cl,(g) 2 NOCI(g) K, 1. x 10-6 р He fills a reaction vessel at this temperature with 11. atm of nitrogen monoxide gas and 16. atm of chlorine gas. Use this data to answer the questions in the table below. yes Can you predict the equilibrium pressure of NOCI, using only the tools available to you within ALEKS? х10 no ? If you said yes, then enter the equilibrium pressure of NOC1 at right. Round your answer to 1 significant digit. atmarrow_forward
- I need help finding the value of X and also verifying after usinf the simplifying assumption to solve for x.arrow_forwardPlease solve thisarrow_forwardA chem gineer is studying the following reaclon! HCN(aq)+NH,(aq) - CN (aq)+NH,(aq) At the temperature the engineer picks, the equilibrium constant K for this reaction is 0.99. The engineer charges ("fills") four reaction vessels with hydrogen cyanide and ammonia, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction vessel compound concentration expected change in concentration HCN 0.84 M O t increase OI decrease O (no change) NH, 1.00 M O t increase O, decrease O (no change) CN 0.42 M O 1 increase O. decrease O (no change) NH, 0.33 M O t increase O, decrease O (no change) HCN 1.01 M O t increase O. decrease O (no change) NH, 1.17 M O t increase O. decrease O (no change) CN 0.25 NM M t increase O. decrease O (no change) NH. 0.16 MM O t increase O.…arrow_forward
- 4. help use sig figs plsarrow_forwardWrite the equilibrium constant expression for the following reaction: Solid calcium carbonate decomposes into solid calcium oxide and carbon dioxide gas. HTML Editor I E E E 目 x x 三 = Vx 12pt Paragraph O wordsarrow_forwardPhosphorus pentachloride gas decomposes to phosphorus trichloride gas and chlorine gas at 250 degrees C with a Kc of 1.80. At equilibrium the concentrations of phosphorus pentachloride, phosphorus trichloride and chlorine gas are 0.40M, 0.12M and 0.12M respectively. If I add 0.125M PCl5 to the reaction flask, what are the concentrations of each gas once equilibrium is reestablished?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY