
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
(25).
Subject :- Chemistry
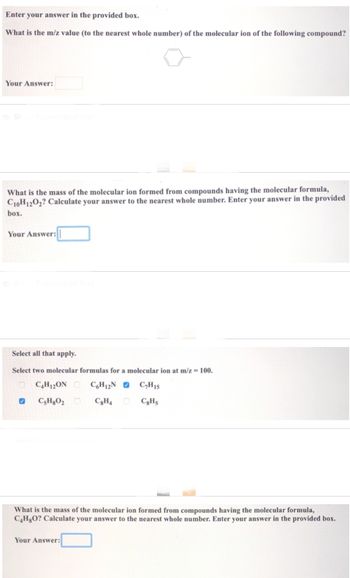
Transcribed Image Text:Enter your answer in the provided box.
What is the m/z value (to the nearest whole number) of the molecular ion of the following compound?
Your Answer:
Show Transcribed Text
What is the mass of the molecular ion formed from compounds having the molecular formula,
C10H12O2? Calculate your answer to the nearest whole number. Enter your answer in the provided
box.
Your Answer:
Select all that apply.
Select two molecular formulas for a molecular ion at m/z = 100.
C4H12ON C6H12N C₂H15
C8H4
C8H5
✔ CsH8O₂
What is the mass of the molecular ion formed from compounds having the molecular formula,
C4H8O? Calculate your answer to the nearest whole number. Enter your answer in the provided box.
Your Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 22-30. (a) The mean free path is the average distance a molecule travels before colliding with another molecule. The mean free path, A, is given by A = kT/(V20P), where k is Boltzmann’s constant, T is temperature (K), P is pressure (Pa), and o is the collision cross section. For a molecule with a diameter d, the col- lision cross section is nd. The collision cross section is the area swept out by the molecule within which it will strike any other molecule it encounters. The magnetic sector mass spectrometer is maintained at a pressure of ~103 Pa so that ions do not collide with (and deflect) one another as they travel through the mass analyzer. What is the mean free path of a molecule with a diam- eter of 1 nm at 300 K in the mass analyzer?arrow_forward(5): The pKa of HF is 3.167. What is Kb for F¯ ?arrow_forwardI do not know how to find the molecular formulaarrow_forward
- (22) 1. NH2 2. AI3 NH2 12 3. NaOH, H,0,A (3) OH. Z.(H9), HCL O KMNO4, NAOH, Darrow_forwardUsing the following monomer: H2C CH C CH- CH CH CH C-F (the benzene ring does not react) draw the mechanism for the formation of the polymer, stopping after attaching three units of the monomer together. DETAIL!! You will need more than one reaction, and yes, this will be one time you need to consider the mechanism. (20 pt.)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY