
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
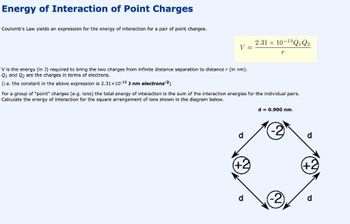
Transcribed Image Text:Energy of Interaction of Point Charges
Coulomb's Law yields an expression for the energy of interaction for a pair of point charges.
V =
V is the energy (in J) required to bring the two charges from infinite distance separation to distance r (in nm).
Q1 and Q₂ are the charges in terms of electrons.
(i.e. the constant in the above expression is 2.31x10-19 J nm electrons-2)
d
For a group of "point" charges (e.g. ions) the total energy of interaction is the sum of the interaction energies for the individual pairs.
Calculate the energy of interaction for the square arrangement of ions shown in the diagram below.
(+2)
2.31 x 10-1⁹Q1 Q2
d
T
d = 0.900 nm.
(+2)
d
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please helparrow_forward5. Rationalize the difference in the atomic and ionic radii of the following species: a) Se (117 pm) and Se²- (198 pm) b) K (231 pm) and K+ (133 pm)arrow_forwardItem 5 Answer the following questions related to the chemical bonding in substances containing Cl. (a) What type of chemical bond is present in the Cl2 molecule? (b) Cl2 reacts with the element Sr to form an ionic compound. Based on periodic properties, identify a molecule, X2, that is likely to react with Sr in a way similar to how Cl2 reacts with Sr. Justify your choice. (c) A graph of potential energy versus internuclear distance for two Cl atoms is given below. On the same graph, carefully sketch a curve that corresponds to potential energy versus internuclear distance for two Br atoms. (d) In the box below, draw a complete Lewis electron-dot diagram for the C2Cl4 molecule. (e) Answer the following based on the diagram you drew above. (i) What is the hybridization of the CC atoms in C2Cl4? (ii) What is the approximate chlorine-carbon-chlorine bond angle in C2Cl4? (iii) Is the C2Cl4 molecule…arrow_forward
- 24. Which of the following atoms and ions is largest: S²-, S, O², CI-, F- (a). Cl (b). F- (c). S (d). S²- (e). 02-arrow_forward17. The electron configuration of indium is [Kr]5s²4d¹⁰5p¹. 4 + How many electrons does a neutral atom of indium have? How does this element complete its octet? Indium forms compounds with chlorine; one example is the formation of InCl (indium (I) chloride). What electron do you think indium gave up? Why?arrow_forwardCompared to their neutral atom counterparts, ions will have smaller or larger atomic radii due to their losing or gaining electrons. According to Jensen (2010), Linus Pauling assigned the radius of the oxygen anion (O“) at 140 pm (larger than the neutral atom, 66 pm). On the other hand, the magnesium atom loses 95 ppm from its 160 ppm radius when it loses two valence electrons, forming the magnesium ion. What is the attractive force in nanoNewtons between the magnesium and oxygen ions? Use 2 decimal places. What is the repulsive force in nanoNewtons between the magnesium and oxygen ions? Use 2 decimal places.arrow_forward
- Match the following electron configurations to the correct atoms or ions. v 1s2s2p6 | 152522p63s²3p6 v 152522p 3s23p 4s2 3a8 v 1522s2p63s²3p2 A. Si В. Са 2+ C. Ni D. Na +arrow_forward11. The ground state electron configuration of 29Cu atom is: eHAr] 4s' 3de b) [Ar] 4s' 4d c) [Kr] 4s' 3dl0 d) [Kr) 4s 3d e) [Ar] 4s' 3d 12. Which of the following isoelectronic ions has the smallest radius: a) Ca b) Mg c) O 13. Which of the following compounds would be expected to bave the highest melting point? d) S- a) NCI, c) MgCla d) LICI e) CCl. 14. Which of these species have two resonance structures? a) CH, b) CH;O e) H2O d) NO,CI e) H;S 15. The formal charge on N in the Lewis structure of NO, is: a) +2 b) +1 d) – 1 -2 16. which of the following compounds does not obey the octect rule? a) SiCL b) XeCle c) PH, d) H;S O NO: 17.What is the molecular geometry (shape) of BrF, ? a) T-shaped d) trigonal pyramidal b) tetrahedral e) seesaw or distorted tetrahedral square planar 18. The hybridization of the central atom, P, in PCla is: O sp b) sp c) sp d) sp'd? e) sp' d 19. Which of the felowing compounds is polar (has a dipole moment)? a) CF. E) BeF, d) XeF, e) BF, 20. According to…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY