
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
! ( plz do all with detail explanation , if you have plan to do only 1 plz skip lets others do all , these are very easy , give ans with explanation )
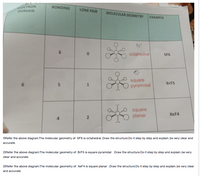
Transcribed Image Text:ELECTRON
DOMAIN
BONDING
LONE PAIR
MOLECULAR GEOMETRY
EXAMPLE
90
octahedral
SF6
square
pyramidal
BRF5
square
XEF4
planar
4
1)Refer the above diagram.The molecular geometry of SF6 is octahedral Draw the structure.Do it step by step and explain.be very clear and
accurate.
2)Refer the above diagram.The molecular geometry of BrF5 is square pyramidal Draw the structure.Do it step by step and explain.be very
clear and accurate.
3)Refer the above diagram.The molecular geometry of XEF4 is square planar Draw the structure.Do it step by step and explain.be very clear
and accurate.
1.
2.
5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Similar questions
- e: 1 hour, 52 minutes, 36 seconds. mpletion Status: Moving to another question will save this response. tion 7 (a) O a Which structure has a formal charge of -1 on the carbon shown with a "C"? Note that the atom of interest in each case O b O C d || =C- (b) d (c) (d) ther question will save this response.arrow_forwardI think answer is F... Explanation?arrow_forwardGive clear handwritten Correct answer pleasearrow_forward
- (e) HBr (2 equiv) Br, Br (f) LOME O3 then Me,s (g) 1) BH3 2) HONAOH/H,O Me H OH D Me Me Me (2)arrow_forward9:44 PM Wed Mar 29 Draw the ester electrophile that would be needed to make this product through a Claisen condensation reaction. O O کہا ہے + Q Problem 60 of 20 Atoms, Bonds and Rings Draw or tap a new bond to see suggestions. Charges Undo X Reset › @ 63% Drag To Pan Remove Done Submit || + | |arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY