
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
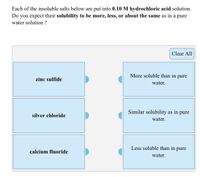
Transcribed Image Text:Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution.
Do you expect their solubility to be more, less, or about the same as in a pure
water solution ?
Clear All
More soluble than in
pure
zinc sulfide
water.
Similar solubility as in pure
silver chloride
water.
Less soluble than in pure
calcium fluoride
water.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Each of the insoluble salts below are put into 0.10M hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All More soluble than in pure lead bromide water. Similar solubility as in pure barium sulfite water. Less soluble than in pure lead fluoride water.arrow_forwardEach of the insoluble salts below are put into 0.10 M hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All More soluble than in pure lead fluoride water. Similar solubility as in pure magnesium carbonate water. Less soluble than in pure lead bromide water.arrow_forwardEach of the insoluble salts below are put into 0.10 M hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All More soluble than in pure calcium carbonate water. Similar solubility as in pure barium fluoride water. Less soluble than in pure silver bromide water.arrow_forward
- Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All More soluble than in pure silver chloride water. Similar solubility as in pure calcium fluoride water. Less soluble than in pure silver sulfite water.arrow_forwardUse the References to access important values if needed for this question. Each of the insoluble salts below are put into 0.10 M hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution? Visited Clear All More soluble than in pure lead fluoride water. calcium sulfite Similar solubility as in pure water. Less soluble than in pure lead bromide water.arrow_forwardEach of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All More soluble than in lead hydroxide pure water. Similar solubility as in silver chloride pure water. nickel(II) sulfide Less soluble than in pure water.arrow_forward
- Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All More soluble than in pure silver sulfite water. Similar solubility as in pure manganese(II) hydroxide water. Less soluble than in pure lead chloride water.arrow_forwardEach of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? lead carbonate barium fluoride Use the References to access important values if needed for this question. lead chloride Clear All More soluble than in pure water. Similar solubility as in pure water. Less soluble than in pure water.arrow_forwardEach of the insoluble salts below are put into 0.10 M hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? lead bromide barium fluoride nickel(II) carbonate Clear All More soluble than in pure water. Similar solubility as in pure water. Less soluble than in pure water.arrow_forward
- Each of the insoluble salts below are put into 0.10 M hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution? magnesium fluoride manganese(II) carbonate lead bromide Clear All More soluble than in pure water. Similar solubility as in pure water. Less soluble than in pure water.arrow_forwardCompare the solubility of silver carbonate in each of the following aqueous solutions: Clear All 0.10 M AgCH3COO More soluble than in pure water. 0.10 M Na2C03 Similar solubility as in pure water. 0.10 M NaNO3 Less soluble than in pure water. 0.10 M NH CH3COOarrow_forwardEach of the insoluble salts below are put into 0.10 M hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? magnesium fluoride lead sulfide lead bromide Clear All More soluble than in pure water. Similar solubility as in pure water. Less soluble than in pure water.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning