Each 15 mL (tablespoon) contains: Potassium Chloride, USP... 20 mEq Inactive Ingredients: citric acid, FD&C Yellow #6, glycerin, methylparaben, natural/artificial orange flavor,propylene glycol, propylparaben, purified water, sodium citrate dihydrate, sucralose. Dosage and Administration: See accompanying prescribing information. Store at 25°C (77°F); excursions permitted to 15°-30°C (50°-86°F). KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. NDC 0603-1542-58 Potassium Chloride Oral Solution, USP, 10% 20 mEq per 15 mL Chloride QUALITEST PHARMACEUTICALS P2/15 PD DILUTE PRIOR TO ADMINISTRATION Rx only 473 mL LEHIGH VALLEY TECHNOLOGIES, INC. ALLENTOWN, PA 1102 Qualitest 3 0603-1542-589 Lot No.: Exp. Date: NO VARNISH
Each 15 mL (tablespoon) contains: Potassium Chloride, USP... 20 mEq Inactive Ingredients: citric acid, FD&C Yellow #6, glycerin, methylparaben, natural/artificial orange flavor,propylene glycol, propylparaben, purified water, sodium citrate dihydrate, sucralose. Dosage and Administration: See accompanying prescribing information. Store at 25°C (77°F); excursions permitted to 15°-30°C (50°-86°F). KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. NDC 0603-1542-58 Potassium Chloride Oral Solution, USP, 10% 20 mEq per 15 mL Chloride QUALITEST PHARMACEUTICALS P2/15 PD DILUTE PRIOR TO ADMINISTRATION Rx only 473 mL LEHIGH VALLEY TECHNOLOGIES, INC. ALLENTOWN, PA 1102 Qualitest 3 0603-1542-589 Lot No.: Exp. Date: NO VARNISH
Chapter21: Heparin Infusion Calculations
Section: Chapter Questions
Problem 1.3P
Related questions
Question
Order: Potassium chloride 30 mEq, PO, daily with food
Available: Potassium chloride 20 mEq/15 mL
How many milliliters of potassium chloride will the patient receive? __ mL
Round to the nearest tenth.
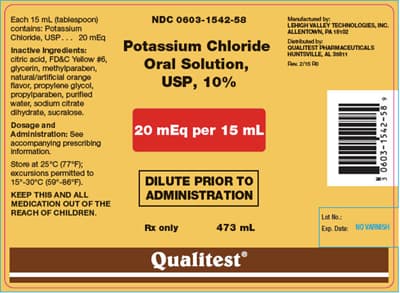
Transcribed Image Text:Each 15 mL (tablespoon)
contains: Potassium
Chloride, USP... 20 mEq
Inactive Ingredients:
citric acid, FD&C Yellow #6,
glycerin, methylparaben,
natural/artificial orange
flavor,propylene glycol,
propylparaben, purified
water, sodium citrate
dihydrate, sucralose.
Dosage and
Administration: See
accompanying prescribing
information.
Store at 25°C (77°F);
excursions permitted to
15°-30°C (50°-86°F).
KEEP THIS AND ALL
MEDICATION OUT OF THE
REACH OF CHILDREN.
NDC 0603-1542-58
Potassium Chloride
Oral Solution,
USP, 10%
20 mEq per 15 mL
Chloride QUALITEST PHARMACEUTICALS
P2/15 PD
DILUTE PRIOR TO
ADMINISTRATION
Rx only
473 mL
LEHIGH VALLEY TECHNOLOGIES, INC.
ALLENTOWN, PA 1102
Qualitest
3 0603-1542-589
Lot No.:
Exp. Date: NO VARNISH
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you


Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage



Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage
