
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:e Web Note made in Microsoft Edge
Chapter 2: EOc
Question 3
[References)
1 pt
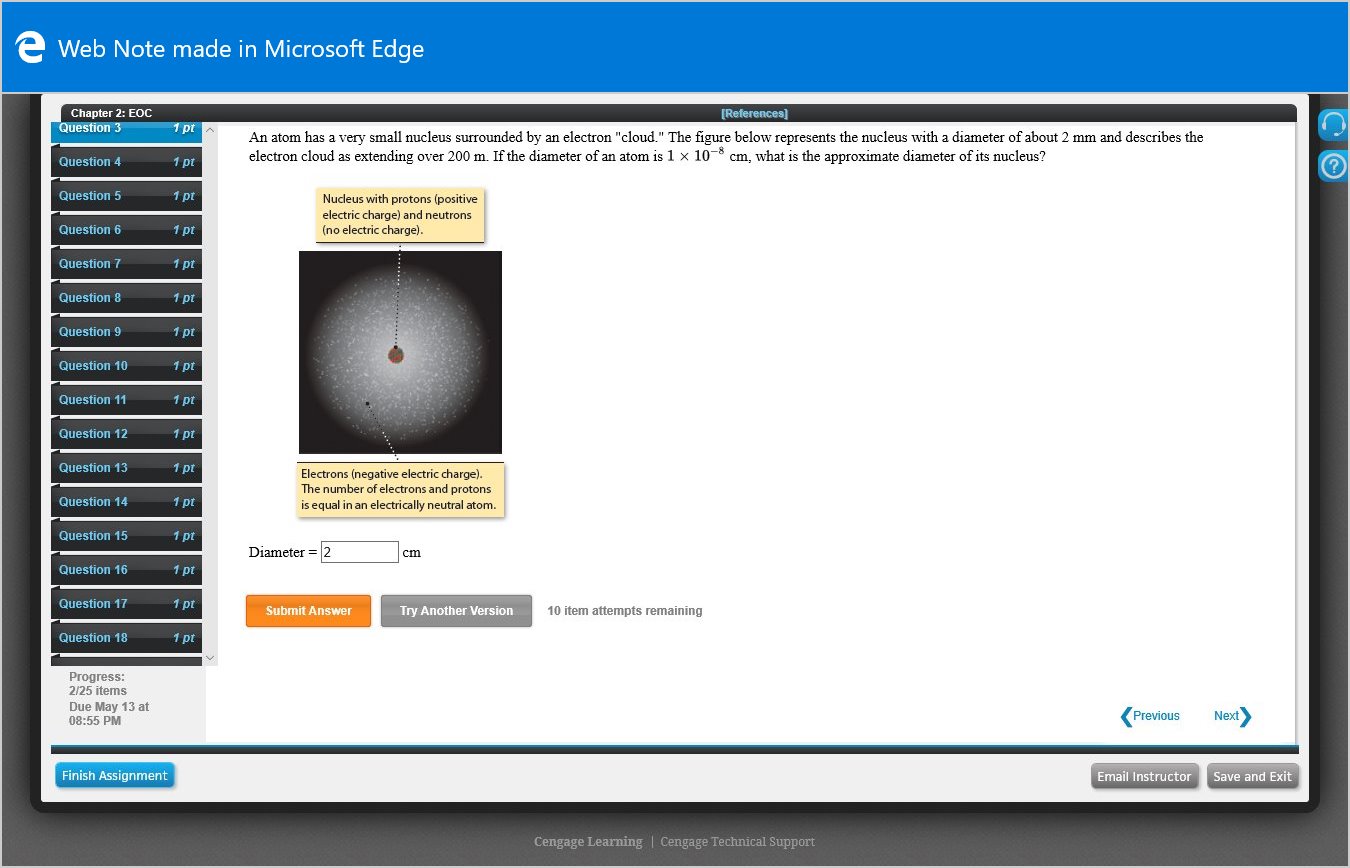
An atom has a very small nucleus surrounded by an electron "cloud." The figure below represents the nucleus with a diameter of about 2 mm and describes the
electron cloud as extending over 200 m. If the diameter of an atom is 1 x 10-8 cm, what is the approximate diameter of its nucleus?
%3D
Question 4
1 pt
Question 5
1 pt
Nucleus with protons (positive
electric charge) and neutrons
(no electric charge).
Question 6
1 pt
Question 7
1 pt
Question 8
1 pt
Question 9
1 pt
Question 10
1 pt
Question 11
1 pt
Question 12
1 pt
Question 13
1 pt
Electrons (negative electric charge).
The number of electrons and protons
is equal in an electrically neutral atom.
Question 14
1 pt
Question 15
1 pt
Diameter = 2
cm
Question 16
1 pt
Question 17
1 pt
Submit Answer
Try Another Version
10 item attempts remaining
Question 18
1 pt
Progress:
2/25 items
Due May 13 at
08:55 PM
Previous
Next
Finish Assignment
Email Instructor Save and Exit
Cengage Learning | Cengage Technical Support
Transcribed Image Text:e Web Note made in Microsoft Edge
Chapter 2: EOC
[References)
Question 1
1 pt
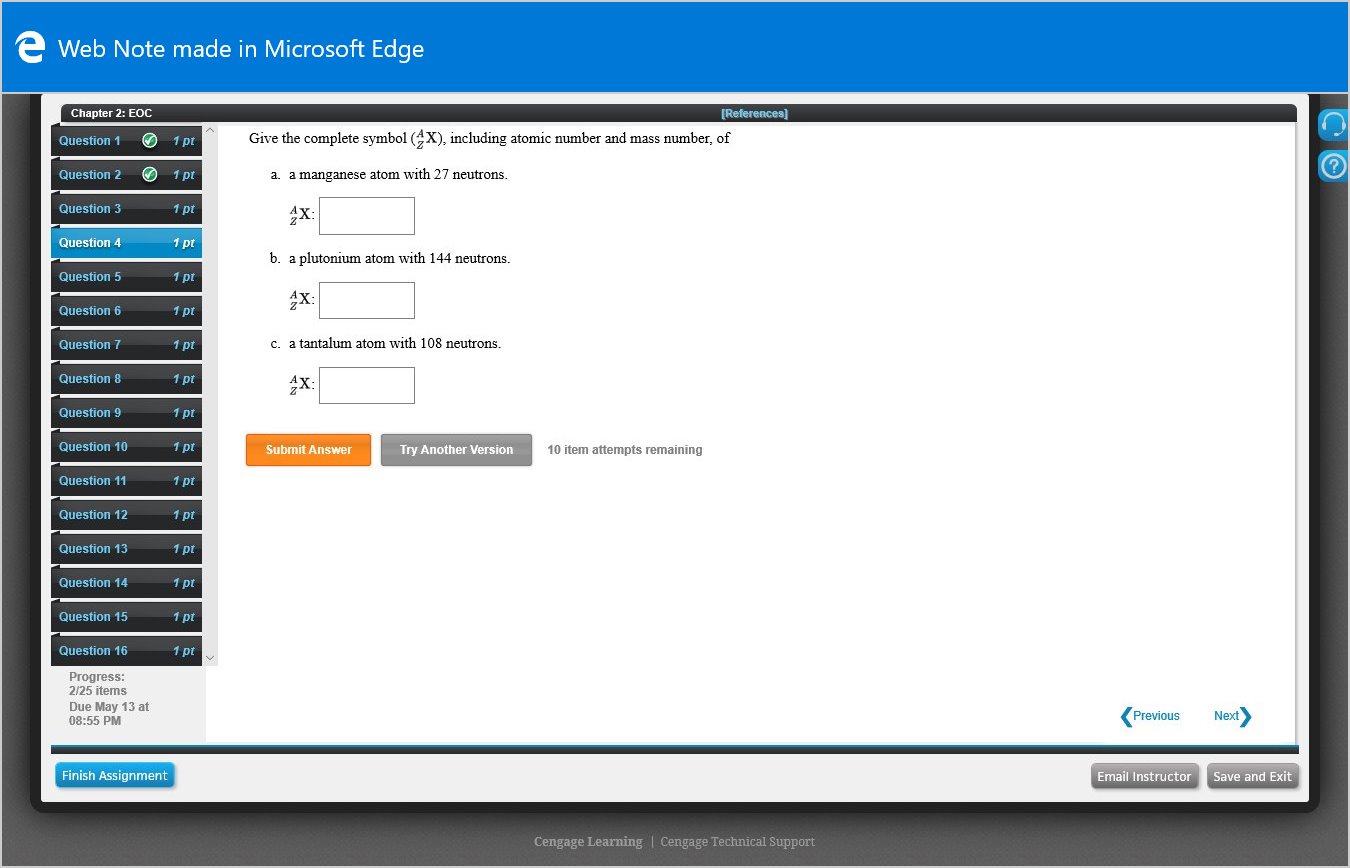
Give the complete symbol (X), including atomic number and mass number, of
Question 2
1 pt
a. a manganese atom with 27 neutrons.
Question 3
1 pt
4X:
Question 4
1 pt
b. a plutonium atom with 144 neutrons.
Question 5
1 pt
X:
Question 6
1 pt
Question 7
1 pt
c. a tantalum atom with 108 neutrons.
Question 8
1 pt
X:
Question 9
1 pt
Question 10
1 pt
Submit Answer
Try Another Version
10 item attempts remaining
Question 11
1 pt
Question 12
1 pt
Question 13
1 pt
Question 14
1 pt
Question 15
1 pt
Question 16
1 pt
Progress:
2/25 items
Due May 13 at
08:55 PM
Next
Previous
Finish Assignment
Email Instructor Save and Exit
Cengage Learning | Cengage Technical Support
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- 22. Elements that have metallic and non-metallic properties are called a. metalloids b.semi-conductors c. non metals d. alloys Sabne buot olo nomme 23. Which element has an atomic mass of 238.03 amu? a) uranium b) plutonium c) radium апо anousis to his bustl d. neptunium 120 24. Which of the following is not a part of John Dalton's atomic theor a. All natter is made of small, indivisible particles called atoms. b. All the atoms of an element are identical in properties such as size and mas c. Atoms of different elements have the same properties. d. Atoms of different elements can combine in specific ways to form new su 25. An ionic bond forms between a metal and non-metal. A. Truearrow_forwardA east.cengagenow.com Chemistry I| X OWLV2 | Online teaching and learning resource from Cengage Learning [References] Use the References to access important values if needed for this Try to complete the missing information for these three isotopes using only the data provided. Isotope #1 • Element Symbol 79 • Nuclear Symbol: Br 35 • Atomic Number • Mass Number • Protons • Neutrons Electrons Isotope #2 • Element Symbol: Fe • Nuclear Symbol • Atomic Number • Mass Number: 55 • Protons • Neutrons • Electrons: 26 Isotope #3 Cengage Learning | Cengage Technical Supportarrow_forwardYou are excited to know from this class that everything in the world is composed of atoms. In addition, an atom looks like the solar system. The _____ are the “planets” in an atom. Group of answer choices a)electrons b)positrons c)neutrons d)protonsarrow_forward
- There are __________ electrons, __________ protons, and __________ neutrons in an atom of plutonium-236. Group of answer choices 236, 236, 94 94, 94, 142 94, 94, 236 142, 142, 94 142, 142, 236arrow_forwardNeed help on part C and part Darrow_forwardArrange the isotopes of sulfur and phosphorus in order of decreasing the number of neutrons.Rank the isotopes from most to fewest neutrons. To rank items as equivalent, overlap them.arrow_forward
- ege.com/course.html?courseld=17576087&OpenVellum HCC Sign in HCC Email Course Home >arrow_forward5. Which of the following particles has the smallest mass? A. Electron B. Proton C. Neutron D. Nucleus 6. How many electrons are in the atom A. 3 B. 6 C. 9 D. 12 12 C 6arrow_forwardGive correct detailed Solution with explanation needed..don't give Handwritten answerarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY