
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
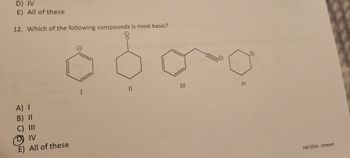
Transcribed Image Text:**Question 12:** Which of the following compounds is most basic?
**Answer Options:**
- A) I
- B) II
- C) III
- D) IV
- E) All of these
**Answer Key:**
- Correct answer: D) IV (This answer is circled)
**Diagrams Explanation:**
1. **Compound I**: A benzene ring with a hydroxyl group (phenol).
2. **Compound II**: A cyclohexanone (six-membered carbon ring with a double-bonded oxygen).
3. **Compound III**: A benzene ring bonded to an ethyl group ending in a triple bond (phenylacetylene).
4. **Compound IV**: An aniline (benzene ring bonded to an amino group, -NH₂).
Compound IV is highlighted as the basic compound due to the presence of the amino group, which is more basic than the other functional groups in the given structures.
**Source:** Fall 2016, Ormrod
Expert Solution
arrow_forward
Step 1
The base which form a stable conjugate acid that is more basic .
-> conjugate acid is formed when base can accepts hydrogen ion.
-> unstable species get hydrogen ion easily.
-> Negative charge on sp2 carbon is unstable .
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7+8arrow_forwardDetermine the pH of water solutions with the following characteristics. Classify each solution as acidic, basic, or neutral. (a) [H3O+] = 7.6 × 10-4 Cacidic Oneutral basic (b) [OH-] = 5.9 × 10-² acidic Oneutral Obasic (c) [H3O+] = [OH¯] acidic Oneutral Obasic (d) [H3O+] = 8.6 × 10-⁹ Oacidic Oneutral Obasic Previous - Nextarrow_forwardWhich of the following compounds is the most acidic? O H20 O о Тон ОН ОНarrow_forward
- Which compound is more acidic?arrow_forwardWhich nitrogen atom in the following molecule cannot be protonated in the presence of an acid? N₂ Select one: O a. Nitrogen a o b. Nitrogen b O c. Nitrogen c O d. Nitrogen d O e. Nitrogen e o f. All nitrogen atoms can be protonated in the presence of an acid. O g. All nitrogen atoms cannot be protonated in the presence of an acid.arrow_forward3. Arrange the following molecules in order of decreasing basicity (from strongest to weakest) ( KOH CO₂Na NaNH, F -SO,Na CH3CH2CH2Na D E Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY