
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Fill in the missing products and intermediates
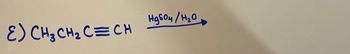
Transcribed Image Text:Набон/ H20
E) CH₂CH₂ C=CH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- the conversion of the amino acid phenylalanine into non-harmful tyrosine is mutated and doesn't work as well (or at all). Phenylalanine will still break down into tyrosine by itself, ol because this is a favorable reaction. Why is the non-functional enzyme a problem? Explain in a few sentences. 3. The disease Phenylketonuria (or PKU) occurs when the enzyme that helps to catalyze O p HOo (pn) O pe oHe avl lls sew.i Snde (a)H noitas on ol woisd sidst nos stolgno ta T b e sd linw ps fertt sro.nalom ni encbeono mundiups sis novi 2noitetnono uTeeg os s oulev THI utinogmet cseo.o 8880 4. Consider the following reaction, which is exothermic: C2H4 (g) + Br2 (g) → C2H¾Br2 (g) ΔΗ< Predict the effect of each of the following perturbations (shift right/products, shift left/reactants, or no effect) using Le Chatelier's principle a. The reaction temperature is increased: b. The reaction volume is decreased: C. More bromine gas is added: d. The reaction is done in the presence of helium: e. C2H4BR2…arrow_forwardComplete the following reaction by providing missing reactants or products as appropriate. (See image)arrow_forwardThe carbonyl group on D-glucose can be reduced to d-glucitol. Identify the reagents that can chrry out this reduction. A.water in acidic solution B. hydrogen with a Platinum catalyst (Hy/Pt) C. Sodium dichromate (NaCr2O) in acidic solution D. Benedict’s reagent E. Enzymesarrow_forward
- Name the first compound on the reactant side of the equation and comment on the way that it is behaving in this reaction.arrow_forwardWe don't see the answer written with a photo or pen, give the answer using the toolarrow_forwardA student was studying enzymes and wanted to track how components can impact the enzymatic activity of amylase on starch. She followed Table 10.1, listed in Lab 10 on page 104. Which of the following statements is true regarding the results of this test? The student has been instructed that something has contributed to the amylase becoming denatured, please indicate the components that could cause the impact the amylase. Question 17 options: The amylase in well B is efficiently working. The amylase in well C is efficiently working. The amylase in select wells has been denatured. The amylase in well A is efficiently working. Wells A, B, and C have enzymes that are denatured. water AgNO3 isopropyl alcoholarrow_forward
- Identify the most likely additional substrates, products, and coenzymes for each reaction in the following imaginary pathway.arrow_forward3. Fill in the box with the major organic product of the first step of the reaction shown? xs CH₂l Ag₂O, Aarrow_forwardFill in the missing intermediates(two), regents(two), and the fill product in the following multi-step synthesis. Assume all reactions proceed too quickly for rearrangements to take placearrow_forward
- The answer choices are as shown in one of the pictures attached. It is the same answer choicesarrow_forwardDraw the structure(s) for the major final product(s) formed in the following reaction sequence. CN s༠༣ Cl, FeCl. 3 H,SO Click and drag to start drawing a structure. lo 用 8 iYB & {EE□arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY