
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
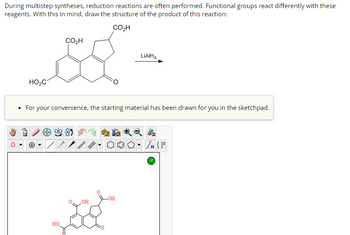
Transcribed Image Text:**Title: Understanding Reduction Reactions in Multistep Syntheses**
During multistep syntheses, reduction reactions are often performed. Functional groups react differently with these reagents. With this in mind, you are tasked with drawing the structure of the product of this reaction:
**Chemical Reaction:**
- **Starting Material:** The structure is an aromatic compound with multiple carboxyl and carbonyl groups. It includes:
- Three carboxylic acid groups (CO₂H).
- One ketone group (C=O) fused into the ring system.
- **Reagent Used:** Lithium aluminum hydride (LiAlH₄) is indicated as the reagent for reduction.
**Instructions:**
- Draw the structure of the product after the reaction with LiAlH₄, considering selective reduction of functional groups.
**Sketchpad Interface:**
- The starting material is displayed in the sketchpad.
- The sketchpad tools include various functional icons such as drawing bonds, rings, atoms, and tools for navigating or modifying the structure.
**Diagram Description:**
- The displayed structure in the sketchpad is identical to the initial structure shown above with carboxylic acid and ketone groups apparent in the aromatic ring system.
**Educational Note:**
Understanding the reactivity and transformation of functional groups like carboxylic acids and ketones with strong reducing agents such as LiAlH₄ is crucial for successfully executing and interpreting multistep synthetic pathways.
Expert Solution
arrow_forward
Step 1: Interpretation of given problem
Given is organic reaction.
LiAlH4 i.e. Lithium aluminium hydride is strong reducing agent.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- nment Score: 0% 01 56 Resources O Hint HR MIN SEC Check Answer ion 1 of 5 If the symbol X represents a central atom, Y represents outer atoms, and Z represents lone pairs on the central atom, the structure Y–X-Y could be abbreviated as XY,Z,. Classify each molecule according to its shape. Linear Bent ( 12) Bent ( 109°) Trigonal T-shaped See-saw Square planar Square руramidal pyramidal Answer Bank XY,Z XY,Z XY,Z, XY,Z, XY,Z XY,Z XY,Zz XY Zarrow_forwardDraw the major organic product for the reaction: Br H20 isopropyl alcoholarrow_forwardComplete these nucleophilic substitution reactions. In each reaction, show all electron pairs on both the nucleophile and the leaving group.arrow_forward
- 1. Alcohol A reacts with oxygen slowly to produce a,ß-unsaturated ketone B. Applying similar reaction conditions to alcohol C, it is expected that ketone D is produced. However, D is not the product of this reaction. What is the actual product formed from the oxidation of C? Explain your reasoning. ܕ ܕܝܪ OH A B ОН 0arrow_forwardHydrocarbon X has the formula C6H12.X reacts with one molar equivalent of hydrogen in the presence of a palladium catalyst to form a product having 12 primary hydrogens.Treatment of X with ozone followed by zinc in aqueous acid gives a mixture two aldehydes.What is the structure of X?arrow_forwardGive the compounds their correct IUPAC names A و بام علا B C EP H ra ne ad E F G D TEF K L Marrow_forward
- Draw structures for the products formed in the following reactions. If more than one product is possible, show all of them, and indicate the major product, if any.arrow_forwardWhat is the major organic product of the following reaction? 01 NH NH AICIarrow_forwardDraw a structural formula for the major organic product of the following reaction: + Br₂ CH₂Cl2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY