
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
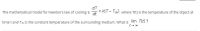
Transcribed Image Text:dT
= k(T- Tm), where T(t) is the temperature of the object at
The mathematical model for Newton's law of cooling is ot
time t and Tm is the constant temperature of the surrounding medium. What is lim T(t) ?
t+ 00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Must solve the whole table. Dont solve it partially. Solve completely with steps.arrow_forward(9 blanks) Derive the work done of an ideal gas undergoing isothermal expansion at temperature T1 if the volume changes from V1 to V2. Express in terms of P1 and P2. V2 W = V1 Where: p= TV By substitution: V2 dv W = vị V Applying the rules of integration, the word done will become W= In| Using: P1V1 = nRT & P2V2 = nRT The work done in terms of pressure is expressed as W = In >arrow_forwardThe p-v diagram all make sense, but the equations are unreadable. i dont understand where some units have come from etc, could you please further explain the equations. thnks, feel free to take the relevant amount of creditsarrow_forward
- This is a question from my problem sheet with the answers. i dont understand how to answer this question. Please use this book for the values: Thermodynamic and Transport Properties of Fluids by Rogersand Mayhew.arrow_forwardA graduate student wants to use van der Waals' equation to express the pressure-volume- temperature relations for a gas. Her project requires a reasonable degree of precision in the p-V-T calculations. Therefore, she made the following experimental measurements with her setup to get an idea of how easy the experiment would be: Temperature, K Pressure, atm Volume, ft'/lb mol 273.1 273.1 200 1.860 1000 0.741 Determine values of constants a and b to be used in van der Waals' equation that best fit the experimental data.arrow_forwardA hot small copper ball [c in kJ/kg.°C, k in W/m.°C] is suddenly placed in water maintained at 100 °C. The convection heat transfer coefficient is h in W/m². ºC. Derive equation to calculate the time required to reduce ball to temperature T.arrow_forward
- The van der Waals (vdW) equation is a cubic equation of state (CEOS) which may be used to calculate the volume or pressure conditions for a real gas: P= a = RT a v-b v² Here, v represents the molar volume of the gas, and P, R, and T' are the absolute pressure, ideal gas constant, and absolute temperature of the gas, respectively. The values a and b are component dependent an may be calculated using: 27 R²T² 64 P C and b= 1 RT 8 Pc So, when both the critical pressure (Pc) and critical temperature (Tc) are known, one can apply the vdW CEOS to give a more accurate calculate more accurate properties than the ideal gas law. One must be careful to use absolute values for the pressure & temperature (e.g., unit of K not °C) and a value for the gas constant that is consistent with the unit desired (such as R = 0.0083144598 L·bar/K·mol) Do the following: a) Calculate the molar volume (L/mol) of nitrogen gas (Pc = 33.96 bar, Tc = 126.19 K) at 5 bar and 30°C using both the ideal gas law & using…arrow_forwardUnit systema) Determine the units of pressure in the equation pv=RT v: specific volume, m3/kg R: constant in kJ/kgK, T: temperature in Karrow_forwardfet), force force, f 10 Newt. m2 f k, 10sec m, = 10 kg, ma= 20 kg , b= 20 Newton m/sec k, = 30 Newton kz = 60 Newton z, and z are measured with respeet to eguilibrium conditions and all imitial conditions are zero, 1) Write the Newton's second Lau for m, amd m2 2) Derive a statespace represrentation of the conditions : eystem fallowing 2a) only z, is measured 26) only z, is measured 2c) (2,-z,) is measured. un der 3) Derive the following transfor fumetione fG) z,(5) Go G,6) ? G6) ? 4) Describe a mechanical device that can be used to implement the external force fee) as shoun'a bove. 5) Using (Csim) function of MATLAB, plot z,(t), 2, (t)- =, (+)] Zz (t) andarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY