
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Draw the structures of the two carbocation intermediates that might form during the reaction of propene (above) with HBr.
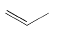
Transcribed Image Text:The image shows a simple structural diagram of an ethene molecule, also known as ethylene. It consists of a linear arrangement with two parallel lines capped at each end with lines extending outward, which represents the double bond between carbon atoms.
### Explanation:
- **Structure**: The diagram illustrates the molecular structure of ethene, where the parallel lines represent a carbon-carbon double bond.
- **Function**: Ethene is an important organic chemical used as a precursor in the production of various polymers and chemicals.
This visual representation is key in understanding molecular geometry and bonding in organic chemistry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Decide which compound is pair is more reactive in electrophilic aromatic substitution reactions. CH3 NH3 (a) or II `NH2 (b) or II (c) I or IIarrow_forwardThe molecule 2-methylbutane undergoes free radical mono-halogenation with bromine. Draw and name the four products that are constitutional isomers.arrow_forwardStep 1: First draw out a carbocation on the C-2 position of butane using the expected empty orbital you would predict. Step 2: Illustrate an example of hyperconjugation using the correct orbital overlap. One example of the process is sufficient. Step 3: Explain why this type of interaction could not occur in a methyl carbocation, using an orbital explanation.arrow_forward
- In each of the organic compound classes given below, write a molecule with a total of 16 carbons (by express formula), with at least one aromatic group and one alkyl group in its structure. Name each structure you write according to the IUPAC system.i) Write and name a molecule of corboxylated acid anhydride.j) Write down and name a half ketal molecule.k) Write and name an N, N-diarylamide molecule.arrow_forwardUsing Zaitsev's rule, choose the most stable alkene among the following. Draw out the structures of each of the options then select the correct answer. Recall that Zaitsev's Rule is about alkene stability. The trend for this is similar to the trend for carbocation stability. Either more or fewer groups on the alkene (and the carbocation) make it more stable. This question is wanting you to remember if it's more or fewer. A) 1,2-dimethylcyclohexene B) 1,6-dimethylcyclohexene C) cis-3,4-dimethylcyclohexene D) They are all of equal stability according to Zaitsev's rule. Provide the major dehydration product of the following reaction. Recall which of the four substitution and elimination reactions a dehydration is. Before you do your S# or EF mechanism, remember what heteroatoms do in the presence of an acid (aka-the-OH is a bad leaving group, but protonating it might turn it into a good one). Don't forget to apply the concept from the previous question. OH Ht Aarrow_forward12. Which of the following carbocations is the least stable one? 13. The heats of complete hydrogenation of the following three hydrocarbons are 105, 116, 275 and 292 (in kJ/mol). Which hydrocarbon does feature the heat of hydrogenation of 292 kJ/mol?arrow_forward
- Draw the structural formula for all the alkenes with the indicated molecular formula that, without undergoing a rearrangement, produce the compound shown as a major product. Br. C7H12 Br2/H₂O OH . You do not have to consider stereochemistry. • If more than one structure fits the description, draw them all. ⚫ Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. 0 ? F ChemDoodle Previous Nextarrow_forward5) What are two way to make the following molecule from benzene? Which one do you think it better? Why?arrow_forwardPlease answer all questions. This is all the informationarrow_forward
- Provide the systematic name for each of the following isomeric esters with the chemical formula C-H1202. (Be sure to indicate double bond stereochemistry using (E)/(Z) notation. Indicate stereochemistry in rings with the terms cis or trans. It is not necessary to use italics in writing compound names.) ball & stick labels ball & stick labels ball & stick + labelsarrow_forwardThe rate law for addition of Br2Br2 to an alkene is first order in Br2Br2 and first order in the alkene. Does this information suggest that the mechanism of addition of Br2Br2 to an alkene proceeds in the same manner as for addition of HBr? Explain.arrow_forwardThis is all of what the question is askingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY