
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
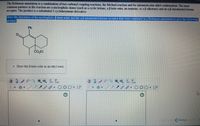
Transcribed Image Text:References
The Robinson annulation is a combination of two carbonyl coupling reactions, the Michael reaction and the intramolecular aldol condensation The most
common partners in the reaction are a nucleophilic donor (such as a cyclic ketone, a B-keto ester, an enamine, or a B-diketone) and an aB-unsaturated ketone
acceptor. The product is a substituted 2-cyclohexenone derivative.
Draw the structures of the nucleophilic 6-ketro ester and theaB-unsaturated ketone acceptor that were conbined in a Robinson annulation to give the following
product
Ph
CO,Et
• Draw the B-keto ester as an ethyl ester,
P.
Activate(Previous Ows
GoithSertinc
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Robinson annulation is a combination of two carbonyl coupling reactions, the Michael reaction and the intramolecular aldol condensation. The most common partners in the reaction are a nucleophilic donor (such as a cyclic ketone, a ß-keto ester, an enamine, or a ß-diketone) and an a,ß-unsaturated ketone acceptor. The product is a substituted 2-cyclohexenone derivative. il The above reaction between a ß-keto ester and an a,ß-unsaturated ketone yields a product containing a 2- cyclohexenone ring. One of the steps of this reaction follows. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions AC↔XT я OEt -I H OEt CO₂Et Eto: يز OEt EtOHarrow_forwardThe Robinson annulation is a combination of two carbonyl coupling reactions, the Michael reaction and the intramolecular aldol condensation. The most common partners in the reaction are a nucleophilic donor (such as a cyclic ketone, a B-keto ester, an enamine, or a B-diketone) and an a,8-unsaturated ketone acceptor. The product is a substituted 2-cyclohexenone derivative. The above reaction between a enamine and an a,B-unsaturated ketone yields a product containing a 2-cyclohexenone ring. One of the steps of this reaction follows. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions H-A A OH HO Nest Emal Instructor Save and Exarrow_forwardThe Robinson annulation is a combination of two carbonyl coupling reactions, the Michael reaction and the intramolecular aldol condensation. The most common partners in the reaction are a nucleophilic donor (such as a cyclic ketone, a β-keto ester, an enamine, or a β-diketone) and an α,β-unsaturated ketone acceptor. The product is a substituted 2-cyclohexenone derivative. Draw the structures of the nucleophilic β-keto ester and the α,β-unsaturated ketone acceptor that were combined in a Robinson annulation to give the following product: Draw the β-keto ester as an ethyl ester,arrow_forward
- The Robinson annulation is a combination of two carbonyl coupling reactions, the Michael reaction and the intramolecular aldol condensation. The most common partners in the reaction are a nucleophilic donor (such as a cyclic ketone, a β-keto ester, an enamine, or a β-diketone) and an α,β-unsaturated ketone acceptor. The product is a substituted 2-cyclohexenone derivative. Draw the structures of the nucleophilic β-diketone and the α,β-unsaturated ketone acceptor that were combined in a Robinson annulation to give the following product:arrow_forwardThe overall reaction produced by the aldol condensation of acetophenone and benzaldehyde is shown below. + NaOH, A Acetophenone Benzaldehyde Chalcone Which of the following steps are involved in the mechanism that leads to the formation of chalcone? он + он H OH он + Он OH + Тон Он он + + Он ОН + онarrow_forwardWhich of the following is a key intermediate for the reaction in the box? A) Benzophenone B) Benzoic acid C) Benzaldehyde D) Phenyl methyl etherarrow_forward
- Write the complete mechanism for the hydroxide ion catalyze reaction of cyclohexanone and 1-methylcyclohexanecarbaldehydearrow_forwardPlease help me answer questions 4 and 5 as seen in the photo!arrow_forwardThe following reactivity order has been found for the saponification of alkyl acetates by aqueous NaOH. Explain. CH3CO2CH3 > CH3CO2CH2CH3 > CH3CO2CH(CH3)2 > CH3CO2C(CH3)3arrow_forward
- Following are the steps in one of the several published syntheses of frontalin, a pheromone of the western pine beetle. (a) Propose reagents for Steps 18. (b) Propose a mechanism for the cyclization of the ketodiol from Step 8 to frontalin.arrow_forwarddraw the products obtained in each of the following reactions.arrow_forwardThe Stork reaction is a condensation reaction between an enamine donor and an α,β-unsaturated carbonyl acceptor. The overall reaction consists of a three-step sequence of formation of an enamine from a ketone, Michael addition to an α,β-unsaturated carbonyl compound, and hydrolysis of the enamine in dilute acid to regenerate the ketone. Consider the Stork reaction between cyclohexanone and propenal Draw the structure of the product of the enamine formed between cyclohexanone and dimethylamine. - Michael addition to an α,β-unsaturated carbonyl compound, and - hydrolysis of the enamine in dilute acid to regenerate the ketone.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT