
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
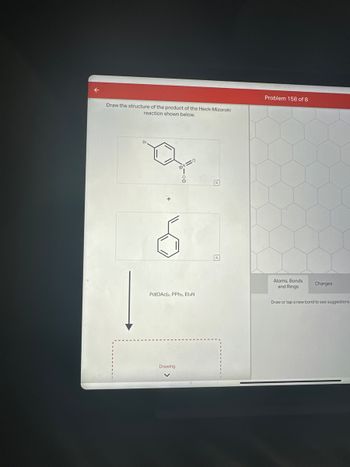
Transcribed Image Text:Draw the structure of the product of the Heck-Mizoroki
reaction shown below.
Br
=0
Pd(OAc)2, PPh3, EtзN
Drawing
Problem 158 of 8
Atoms, Bonds
and Rings
Charges
Draw or tap a new bond to see suggestions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Draw the major organic product of the reaction of aniline with excess methyl iodide. Include any lone pairs of electrons and nonzero formal charges. Draw only one compound. Your product should contain a benzene ring. „NH2 excess CH31arrow_forwardChapter 8 Problem 79 ▼ Part A Which of the following compounds is/are the products of the following reaction? H H CH3 Br I. + HO Submit CH3 heat II. I only O II only OI and II are of equal yield. O I is minor, Il is major. O I is major, II is minor. ? CH3 Previous Answers Request Answer X Incorrect; Try Again; 8 attempts remainingarrow_forwardQuestion 9 Please predict the products for each of the following reactions: A ABCD 1. Na 2. PrBr เวรเ Py (pyridine) Na 10 9 OH 18 1. TsCI, Py 2. NaBr, DMSO SOCI₂, Py H-N'+ CrO3Cl PCC Na2Cr2O7 H2SO4 HCI ZnCl2 2. KI, DMF 1. MsCI, Et N (base) B с D Na NO REACTIONarrow_forward
- Part A Predict the major organic product formed when the compound shown below undergoes a reaction with EtONa. Interactive 3D display mode H₂C Br ww Review Constants I Periodic Table CH 3 CH3 Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. H: 120 I UZOarrow_forward10:08 AM Thu Mar 23 Problem 33 of 23 Br2 (1 equiv) Draw the product of the reaction shown below. Ignore inorganic byproducts. FeBr3 ☎ @ : 66% Select to Draw Submitarrow_forwardPart A Provide the major organic product of the following reaction. KOC(CH3)3 Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. 0 2 H± 20 1 L EXP. CONT. ? L H Br [1] Α H 0 N ○ S Marvin JS by ChemAxon Br - PF Aarrow_forward
- for the reaction 2NH3 - > 3H2(g) + N2(g) the equilibrium concentrations were found to be [NH3] = 0.250M, [H 2] = 0.470M and [N2] = 0.800 M. What is the equilibrium constant for this reaction?arrow_forwardCan you solve allarrow_forward+ Draw the product of the reaction shown below at physiological pH (pH = 7.4). Ignore inorganic byproducts. N- H H&N 00 (BOC)20 EtsN Drawing Atoms, Bonds and Rings Charges .OH NH HN mo Undo Re Remove Doarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY