
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
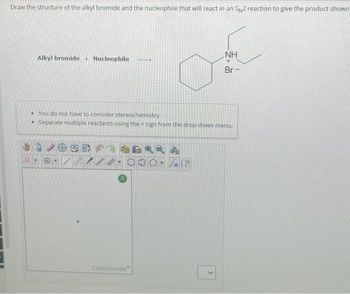
Transcribed Image Text:Draw the structure of the alkyl bromide and the nucleophile that will react in an S2 reaction to give the product shown
Alkyl bromide + Nucleophile
O-
• You do not have to consider stereochemistry.
Separate multiple reactants using the + sign from the drop-down menu.
-
-85
T
ChemDoodle
Y
SIF
NH
Br-
>
-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Butanone undergoes a nucleophilic addition with a Grignard reagent made from 1-bromopropane and magnesium metal in THF solution. The alkoxide formed from the nucleophilic addition is then conveted into the final product by the careful addition of dilute acid. Complete the mechanism by following the instructions provided for each step. Step 1. Nucleophilic Addition in THFarrow_forwardSuppose you were told that each reaction is a substitution reaction, but you were not told the mechanism. Describe how you could conclude from the structure of the haloalkane or cycloalkene, the nucleophile, and the solvent that each reaction is an SN1 reaction.arrow_forwardFill in the reagents or synthetic intermediates in the following scheme. 1) NaOEt / EIOH 2) H,0arrow_forward
- Predict the starting alkyl bromide that would produce the product shown in the SN1 reaction. Assume no rearrangements. Stereochemistry is not considered. Draw Alkyl Bromide Reactant CH3CH2OHarrow_forwardDraw a reaction with reactant(s) and expected product(s) for each of the 6 alkyl halides in the SN1 reaction. If no product formed for any of the reactions indicates no reaction . In SN1 1% silver nitrate in ethanol was used to react with 6 alkyl halids 1-chlorobutane, 2-chlorobutane, Allyl chloride, 2-chloro-2-methylpropane, 1-chloro-2-methylpropane, 2-bromobutanearrow_forwardAre phenyl carbocations more stable than tertiary carbocations? Is there a carbocation that is more stable than a tertiary carbocation? If yes, what is the reason why it is more stablearrow_forward
- Draw a reaction with reactant(s) and expected product(s) for each of the 6 alkyl halides in the SN2 reaction. If no product formed for any of the reactions indicates no reaction . In SN2 12% sodium iodide in acetone was used to react with 6 alkyl halids 1-chlorobutane, 2-chlorobutane, Allyl chloride, 2-chloro-2-methylpropane, 1-chloro-2-methylpropane, 2-bromobutanearrow_forwardDemonstrate the results of the reactions between this alkyne and the various reagents.arrow_forward7. Complete the following SN2 reaction by providing structures for the necessary starting materials. H + Na Clarrow_forward
- The SN1 mechanism starts with the rate-determining step which is the dissociation of the alkyl halide into a carbocation and a halide ion. The next step is the rapid reaction of the carbocation intermediate with the nucleophile; this step completes the nucleophilic substitution stage. The step that follows the nucleophilic substitution is a fast acid-base reaction. The nucleophile now acts as a base to remove the proton from the oxonium ion from the previous step, to give the observed product. Draw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all nonzero formal charges.arrow_forwardWhat type of reaction is this? Br + Br + H2О O SN1 O SN2 O E1 O E2 O Nucleophilic aromatic substitutionarrow_forwardIF esize this compound by the → t-butyl ethyl ether Show the steps necessary to synthesize this compound by a rignard reaction. Start with an alkyl halide; after that you can add any organic or inorganic compound. → 1-hexanol Consider the following compounds:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY