
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![**Transcription for Educational Website**
---
### Task: Draw the Structural Formula for the Ionic Form of the Following Phosphate Ester
**Chemical Structure**
The chemical structure shown above is a phosphate ester with the following formula:
\[ \text{H}_3\text{C} - \text{O} - \text{P} = \text{O} - \text{O} - \text{CH}_3 \]
- The phosphate group (\( \text{PO}_4 \)) is central, with one oxygen double-bonded to phosphorus and two oxygen atoms single-bonded to phosphorus.
- Two methyl groups (\( \text{CH}_3 \)) are attached via oxygen atoms.
**ChemDoodle Tool Interface**
The image includes a ChemDoodle interface with various drawing tools:
- **Tool Icons:** These are located at the top and include functions such as drawing lines, rings, erasers, undo/redo, and selection tools.
- **Drawing Area:** The main workspace for creating chemical structures.
- **Help Icon:** Provides information or troubleshooting for using the ChemDoodle software.
**Current Workspace Contents**
- The workspace currently contains the text "CH₄", indicating the placement of a methane molecule.
**Instructions**
- Use the provided tools in ChemDoodle to manipulate and draw the complete ionic form of the phosphate ester.
- Consider charge distribution and resonance structures that might be relevant for the ionic form.
---
This transcription aims to assist students in using ChemDoodle for drawing chemical structures by explaining the interface and tools available.](https://content.bartleby.com/qna-images/question/fc2fcf10-1c06-45bd-a437-ad10722d937a/96833562-9c3e-4bad-b1e8-27f7f7c1a60e/hwzxew_thumbnail.png)
Transcribed Image Text:**Transcription for Educational Website**
---
### Task: Draw the Structural Formula for the Ionic Form of the Following Phosphate Ester
**Chemical Structure**
The chemical structure shown above is a phosphate ester with the following formula:
\[ \text{H}_3\text{C} - \text{O} - \text{P} = \text{O} - \text{O} - \text{CH}_3 \]
- The phosphate group (\( \text{PO}_4 \)) is central, with one oxygen double-bonded to phosphorus and two oxygen atoms single-bonded to phosphorus.
- Two methyl groups (\( \text{CH}_3 \)) are attached via oxygen atoms.
**ChemDoodle Tool Interface**
The image includes a ChemDoodle interface with various drawing tools:
- **Tool Icons:** These are located at the top and include functions such as drawing lines, rings, erasers, undo/redo, and selection tools.
- **Drawing Area:** The main workspace for creating chemical structures.
- **Help Icon:** Provides information or troubleshooting for using the ChemDoodle software.
**Current Workspace Contents**
- The workspace currently contains the text "CH₄", indicating the placement of a methane molecule.
**Instructions**
- Use the provided tools in ChemDoodle to manipulate and draw the complete ionic form of the phosphate ester.
- Consider charge distribution and resonance structures that might be relevant for the ionic form.
---
This transcription aims to assist students in using ChemDoodle for drawing chemical structures by explaining the interface and tools available.
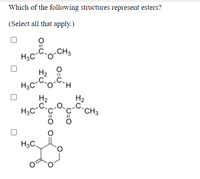
Transcribed Image Text:**Question:**
Which of the following structures represent esters?
(Select all that apply.)
**Structures:**
1. First Structure:
- A molecule with the formula:
- CH₃C(=O)-O-CH₃
- Contains a carbonyl group (C=O) adjacent to an oxygen atom, which is bonded to an alkyl group.
2. Second Structure:
- A molecule with the formula:
- CH₃CH₂C(=O)-O-CH₂H
- Contains a carbonyl group (C=O) adjacent to an oxygen atom, which is bonded to another carbon chain.
3. Third Structure:
- A molecule with the formula:
- CH₃C(=O)-O-CH₂CH₃
- Contains a carbonyl group (C=O) adjacent to an oxygen atom, which is bonded to an ethyl group.
4. Fourth Structure:
- A cyclic molecule with two carbonyl groups (C=O) connected to a shared oxygen atom, forming a lactone-like structure.
**Instructions:** Select all structures that are esters.
**Explanation:**
- An ester is characterized by a carbonyl group (C=O) directly bonded to an oxygen atom (O), which is further bonded to an alkyl or aryl group.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- ORGANIC FUNCTIONAL GROUPS Understanding the names of carboxylate salts What is the systematic name of the product P of this chemical reaction? HO 0 OH O OH OH + NaOH + H₂Oarrow_forwardWhat's the name of the following ester? CH 3CH 2OCOCH 3arrow_forwardName the product of the following reaction:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY