
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
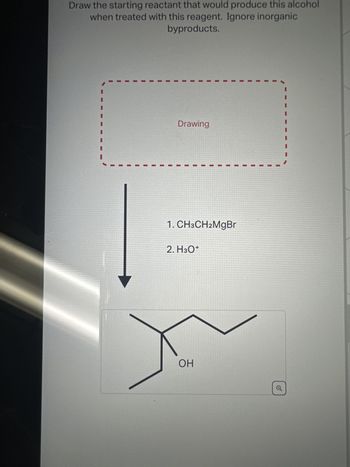
Transcribed Image Text:Draw the starting reactant that would produce this alcohol
when treated with this reagent. Ignore inorganic
byproducts.
Drawing
1. CH3CH2MgBr
2. H3O+
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Draw organic product H3C. + H₂SO4 CH3arrow_forwardDraw the organic product(s) of the following reaction. 1. K2CO3 H. 2. distillarrow_forwardIf the following structure undergoes oxidation reaction, which of the product(s) will form: ОН H2C–CH2-CH2 CH3 O A. Aldehydes only O B. This molecule cannot go through oxidation reaction. OC. Ketone O D. Aldehyde & Carboxylic acid O E. Ether and waterarrow_forward
- 2. YATEMBRO MADRO JAREMBO BESMHO OH Start from acetylene (HC=CH) to make this alcohol. Use any other appropriate organic or inorganic reagents.arrow_forwardWhich of the following is a tertiary alcohol? HC CH H.C HC OI O III OIV CH HO H₂C H₂C H₁C H.C CH H₂ CH CH CH H.C CH "CH, COH IV H.C CH OH H,C CH₂ CH, OHarrow_forwardA mixture of 0.239 g of H2, 1.22 g of N2, and 0.837 g of Ar is stored in a closed container at STP. Find the volume (in L) of the container, assuming that the gases exhibit ideal behavior.arrow_forward
- 1. When an aldehyde is reduced with hydrogen (H2) gas in the presence of a transition metal catalyst, what type of product is formed? A. primary alcohol B. secondary alcohol C. tertiary alcohol D. carboxylic acidarrow_forwardGive three reasons why ethers make good laboratory reagentsarrow_forwardWhich of these is an alcohol? A) C) E) ZI -N₁ -S OH -ОН "CH3 B) D) F) H IZ CH3 -Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY