
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Draw the simplest mechanism possible for the reaction below. You may need to re-draw structures to show bond lines or lone pairs.
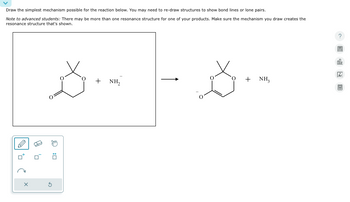
Transcribed Image Text:Draw the simplest mechanism possible for the reaction below. You may need to re-draw structures to show bond lines or lone pairs.
Note to advanced students: There may be more than one resonance structure for one of your products. Make sure the mechanism you draw creates the
resonance structure that's shown.
ப:
S
+
NH₂
+
NH3
Ar
B:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 1 steps with 3 images

Knowledge Booster
Similar questions
- Last person who helped was incorrect it needs to work for mastering Also are the arrows single or double headed Doesn't help when its sloppy drawn thank youarrow_forwardThe first and third boxes are correct. The second(middle box) step is incorrect. Can someone help me figure out the middle box.arrow_forwardPropose a mechanism that accounts for the formation of this product. Br Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where need flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created.arrow_forward
- Draw the structure of all products of the mechanism below. H. H :0: CH3 H H Click and drag to start drawing a structure.arrow_forwardStep 4b: Add curved arrows to show ethoxide deprotonating the most acidic proton. Select Draw Rings More Erase / | c H0 H H :0 : H H H Harrow_forwardFor the following intermediates , please draw as many resonance contributors that you can come up with. Then circle the highest contributor and draw a hybrid resonance illustration for each molecule.arrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardi,ii,iii please.arrow_forwardPlease label the organic and inorganic species so I can know which answer is to what part of the question.arrow_forward
- The mechanism for the following reaction occurs in a stepwise fashion. мол + он + но Draw the correct mechanism in the space below. Be sure to add lone pairs and charges to structures as needed. Ö Add/Remove step х он Click and drag to start drawing a structure.arrow_forwardOH CH3 H2O CH3 (show mechanism) H2SO4 CH3 CH2arrow_forward3.) For the following intermediates, please draw as many resonance contributors that you can come up with. Then circle the highest contributor and draw a hybrid resonance illustration for each molecule.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY