
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw the scheme of reaction for the multi-step synthesis shown in figure 1
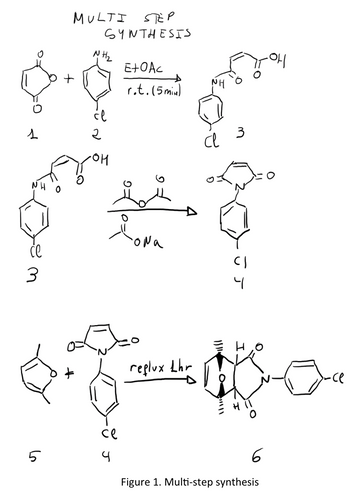
Transcribed Image Text:1
NH
се
3
5
MULTI STEP
SYNTHESIS
+
NH₂
će
2
се
4
E+OAc
r.t. (5 min)
"ona
reflux thr
NH
cl
3
(1
4
но
6
Figure 1. Multi-step synthesis
·ce
Expert Solution
arrow_forward
Step 1
The first step is the -acylation of p-chloroaniline (2) with maleic anhydride (1) to give the compound 3.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H₂C H H₁C Br NBS CH, CH₂ CC, hv **You may assume that Br-Br is formed by a side reaction that occurs (which we discussed in class). This is useful for one of the steps of the mechanism.arrow_forwardConsider the mechanism of the reaction shown below. Give the structure of the next important organic reaction intermediate along the reaction coordinate. Your answer could be the final produ HBr Edit Click on the drawing box above to activate the MarvinSketch drawing tool and then draw your answer to this question. If there is no reaction, then check the "no reaction" box below. no reactionarrow_forwardDraw the complete, detailed mechanism for the reaction shown here and, using the mechanism, predict the major product. TSOH is a strong acid. -NH2 НО ? TSOH, C6H6, Aarrow_forward
- Bha Please don't provide the handwriting solutionarrow_forwardCan i see the mechanism for this reactionarrow_forwardIf H* is eliminated from the carbocation shown here in an electrophile elimination step, then three possible constitutional isomers can form. Draw the mechanism for the formation of all three of those products. H2O + ?arrow_forward
- The reaction shown below produces one alcohol as the major product. Draw that product and show a complete mechanism for its formation using the curved arrow notation. Hint: consider the stability of the carbocation intermediate. H3C CH3 H₂O; trace H₂SO4arrow_forwardRank the following substrates in order from slowest Sy2 reaction rate to fastest. Br H3C Drag answer here fastest Br Drag answer here second slowest Br Drag answer here slowest Br second fastest Drag answer herearrow_forwardDraw the E1 and Sn1 mechanism and the possible productsarrow_forward
- can you ecplain the steps?arrow_forwardIn the given synthetic transformation, which basic reaction type best describes the two reactions? (The number in the answer corresponds to the number of reactions) 1. H2SO4, H2O 2. CrO3, py Step 1: choose your answer... Step 2: choose your answer... choose your answer... Substitution Next Proton transfer Addition Elimination Oxidationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY