
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
please help with arrows and step-by-step detailed answers.. thank you
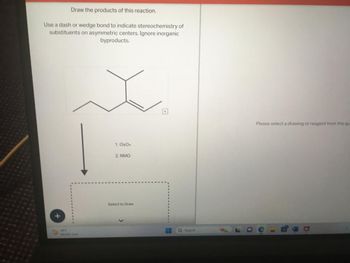
Transcribed Image Text:Draw the products of this reaction.
Use a dash or wedge bond to indicate stereochemistry of
substituents on asymmetric centers. Ignore inorganic
byproducts.
19%
1. 0804
2. NMO
Select to Draw
#
Q Search
Please select a drawing or reagent from the qu
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (SELECT ALL THAT APPLY) Self-awareness theory is based on the idea that O you are the thinker, separate and apart from your thoughts O you are not your thoughts, but the entity observing your thoughts O you are your thoughts O you, as a thinker, cannot be separated from your thoughtsarrow_forwardCan you help me on questions 2 AND 3arrow_forwardM Apple Google Disney ESPN Yahoo! Biomedical Careers Program Apple iCloud Yahoo Images Bing Google Wikipedia COWLv2 |... b D2L ☆ & G For the reaction r r Submit Answer prod03-cnow-owl.cengagenow.com Fe(s) + 2HCI(aq)→→→FeCl₂(s) + H₂(g) AH° = -7.4 kJ and AS° = 107.9 J/K D2L D2L D2L Facebook Twitter LinkedIn Use the References to access important values if needed for this question. The Weather Channel Yelp TripAdvisor b D2L M G C The maximum amount of work that could be done when 2.08 moles of Fe(s) react at 286 K, 1 atm is Assume that AH° and ASº are independent of temperature. Retry Entire Group 4 more group attempts remaining kj. +88 Marrow_forward
- Please create a caption for this table. Solution NaCl Conc. (%) Osmolality (mOsm) % transmittance Absorbance % hemolysis % crenation C distilled 0 0 0.001029 4.987584625 100 0.03354 1 0.177179111 54.61 0.001551 4.809388202 96.42720001 0.05837 2 0.297126222 91.58 0.01012 3.994819487 80.09527231 0.08444 3 0.442542222 136.4 3.849 1.414652089 28.3634704 0.134 4 0.590164444 181.9 64.8 0.188424994 3.777880643 0.2125 5 0.74752 230.4 95.64 0.019360433 0.388172513 0.3368 6 0.89644 276.3 99.56 0.001915112 0.038397585 0.5336 7 1.095648889 337.7 99.98 8.68676E-05 0.001741676 0.9834 8 1.336711111 412 100 0 0 2.1 9 1.755568889 541.1 100 0 0 7.9 10 2.674395556 824.3 100 0 0 57.83 11 4.490211111 1384 100 0 0 99.72arrow_forward7. Chemical Formula: C3H7NO₂ 1H, broad 12 10 2H, broad Final Answer 8 PPM 6 1H q A- 3H d 2 0arrow_forwardI need help to answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY