
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Can you please show all details to understand
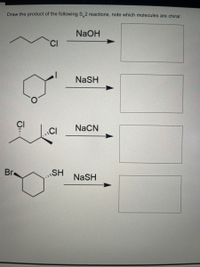
Transcribed Image Text:**Title: Understanding S<sub>N</sub>2 Reactions and Chirality**
---
**Introduction**
In this exercise, we will explore S<sub>N</sub>2 reactions and identify chiral molecules formed as products.
---
**Problem 1:**
- **Reactant:** 1-chlorobutane
- **Reagent:** NaOH
- **Product:** Draw the structure and identify chirality.
The S<sub>N</sub>2 reaction involves the nucleophilic substitution of the chlorine atom by the hydroxide ion. The product will be butanol, which is not chiral due to the presence of a symmetric carbon chain.
---
**Problem 2:**
- **Reactant:** 4-iodotetrahydropyran
- **Reagent:** NaSH
- **Product:** Draw the structure and identify chirality.
The S<sub>N</sub>2 reaction replaces the iodine atom with an SH group. This substitution creates a new chiral center at the reaction site, making the product potentially chiral depending on its stereochemistry.
---
**Problem 3:**
- **Reactant:** 2,3-dichlorobutane
- **Reagent:** NaCN
- **Product:** Draw the structure and identify chirality.
In this reaction, the S<sub>N</sub>2 mechanism replaces one of the chlorine atoms with a CN group. The molecule can have stereoisomers, thus resulting in chiral compounds depending on which chlorine is replaced.
---
**Problem 4:**
- **Reactant:** Bromocyclohexyl thiol
- **Reagent:** NaSH
- **Product:** Draw the structure and identify chirality.
The bromine atom is replaced by an SH group through the S<sub>N</sub>2 mechanism. The chirality will depend on the original configuration of the substituents and their spatial arrangement.
---
**Conclusion**
S<sub>N</sub>2 reactions are highly stereospecific, often resulting in inversion of configuration at the site of substitution. Identifying chirality in the products requires evaluating changes in stereochemistry and the spatial arrangement of substituents.
For further study, ensure to practice drawing the molecular structures carefully to understand the stereochemical outcomes in different S<sub>N</sub>2 reactions.
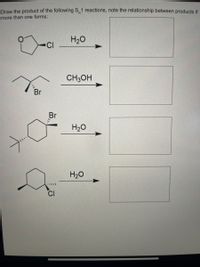
Transcribed Image Text:### Educational Content on S<sub>N</sub>1 Reactions
This section covers the prediction of products for given S<sub>N</sub>1 reactions and understanding the relationships between multiple products, if applicable.
#### Reactions and Mechanisms:
1. **Cyclohexyl Chloride Reaction**
- **Reactants:** Cyclohexyl chloride with water (H<sub>2</sub>O).
- **Products:** Draw the alcohol formed by substitution of the chlorine atom with a hydroxyl group (OH).
2. **Bromocyclohexane Reaction**
- **Reactants:** 2-bromo-2-methylpentane with methanol (CH<sub>3</sub>OH).
- **Products:** Illustrate the ether formed by substitution of the bromine with a methoxy group (OCH<sub>3</sub>).
3. **Tertiary Bromo Compound Reaction**
- **Reactants:** 2-bromo-3,3-dimethylpentane with water (H<sub>2</sub>O).
- **Products:** Represent the alcohol formed by substitution of the bromine with a hydroxyl group (OH).
4. **Substituted Cyclohexyl Chloride Reaction**
- **Reactants:** Chlorocyclohexane with a methyl group at the 4-position, reacting with water (H<sub>2</sub>O).
- **Products:** Show the alcohol formed by substitution of the chlorine atom with a hydroxyl group (OH).
#### Explanation of Reaction Details:
Each of these reactions proceeds through the S<sub>N</sub>1 mechanism, which involves:
- **Formation of a Carbocation Intermediate:** The leaving group (Cl or Br) departs, forming a carbocation.
- **Nucleophilic Attack:** The nucleophile (H<sub>2</sub>O or CH<sub>3</sub>OH) attacks the carbocation to form the final product.
Note any rearrangements or alternative products due to carbocation stability during this process. If more than one product is possible, indicate the stereochemical or structural relationship between them.
This content provides foundational knowledge for organic chemistry courses, focusing on reaction mechanisms and product prediction in nucleophilic substitution reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A bbhosted.cuny.edu/webapps/assessment/take/take.jsp?course_assessment_id=_1... R Paused E Apps G Gmail O YouTube O Maps Home Take Test: Test #1 * Question Completion Status: A MOving to the next question prevents cnanges to tnis answer. Question 4 of 20 > Question 4 4 points Save Answer What is the density of a solid sample that increased the volume of 25.2 mL of water to 34.9 mL when placed in the graduated cylinder and weighs 13.34 g? O A. 0.52 g/mL O B. 0.38 g/mL OC. 1.37 g/mL O D.0.73 g/mL A Moving to the next question prevents changes to this answer. Question 4 of 20 13,846 MAR étv S esc F2 F3 F4 F5 F6 F7 @ %23 24 2 & Q W E R Y IIarrow_forwardExplain process of doping with example.arrow_forwardMyCSU - Columbus State Univer. x | № Inbox - bailes_amber@columbu: x D2L Homepage - Columbus State Un X - со A ALEKS-Amber Bail www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lJgXZp57itWHhRgilODc5MqvhZbKYx2-U-037007TYd Gmail YouTube Maps MyCSU - Columbus... Homepage - Georg... Microsoft Office Ho... B Lesson 2 Disc O CHEMICAL REACTIONS = Solving for a reactant using a chemical equation Ammonia (NH3) chemically reacts with oxygen gas (0₂) to produce nitric oxide (NO) and water (1₂0). What mass of ammonia is consumed by the reaction of 9.9 g of oxygen gas? Be sure your answer has the correct number of significant digits. x10 g ANAKKALE X S ? Email 4 Jessy Vseforestainty a jedan den so $45******arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY