
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:x
✓5
Br
6
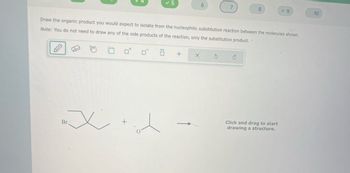
Draw the organic product you would expect to isolate from the nucleophilic substitution reaction between the molecules shown.
Note: You do not need to draw any of the side products of the reaction, only the substitution product.
Ö
+ X
S
8
C
9
Click and drag to start
drawing a structure.
10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction. NH2 + Br. + X 5 Click and drag to start drawing a structure.arrow_forwardDraw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction. Cl + + OH Click and drag to start drawing a structure. +arrow_forwardHow does gold help to catalyze the reaction in the gold catalysis experiment? It deprotonates the methanol, making methanol a better nucleophile. оа. o b. It donates electron density to the alkyne, making the alkyne a better nucleophile. О с. It coordinates to the methanol, making methanol a better nucleophile. o d. It coordinates to the alkyne, making the alkyne a better electrophile.arrow_forward
- Predict the major product of this organic reaction: H+ + H₂O ? Specifically, in the drawing area below draw the skeletal ("line") structure of the major product. If there is no reaction, check the No reaction box under the drawing area.arrow_forwardUse a sheet of paper to answer the following question. Take a picture of your answers and attach to this assignment. Draw the mechanism for the reaction of acetyl chloride (ethanoyl chloride) with the ethoxide anion to yield ethyl acetate (ethyl ethanoate). OCH₂CH3 H₂C+ CI H3C + OCH₂CH3arrow_forward1A. Explain why solvents such as anhydrous diethyl ether or anhydrous tetrahydrofuran (THF) are not reactive with Grignard reagents. 1B. Draw the first step of the reaction mechanism when the Grignard reagent attacks benzophenone before the alkoxide is protonated to form the alcohol. In the mechanism, identify the partially positive and partially negative atoms in the reactants. Explain why these atoms have a partial charge. 1C. Write the mechanism for the reaction of phenyl magnesium bromide with 1) water and 2) acetone.arrow_forward
- i need the answer quicklyarrow_forwardDraw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction. o* o Br SH Ö + X Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? NH2 +R + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed.arrow_forward
- b. A chemist wants to use a Grignard reaction to make 1-phenylethanol. Which of the following reactions will successfully form the product? Assume a final acidic workup a each. For any reactions that will not make the product correctly, propose an alternate way to get to the product. Reaction 1 Reaction 2 CH₂Br Br Mg ether OH Mg ether H Harrow_forwardDraw the principal organic product for the reaction when 2-bromopropane is treated with lithium in diethyl ether, followed by copper(I) iodide and then 1-bromoethane. Click and drag to start drawing a structure. Xarrow_forward29 minutes, 42 seconds. Question Completion Status: A Moving to another question will save this response. Question 15 What is not an expected product of the following allylic substitution reaction? NBS, hv Br Br Compounds II and II Compound II only O Compound I only O Compound II only A Moving to another question will save this response O O Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY