
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
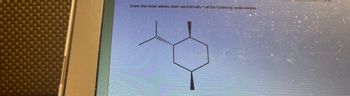
Transcribed Image Text:### Cycloalkane Chair Conformation Explanation
**Problem Statement:**
Draw the most stable chair conformation of the following cyclohexane.
**Diagram Description:**
The image shows a molecular structure that includes a cyclohexane ring with six carbon atoms. Attached to the ring are various substituents, including a methyl group and a position indicating an axial substituent.
**Understanding Chair Conformations:**
Cyclohexane can adopt several conformations, but the most stable form is the chair conformation due to minimized steric hindrance and torsional strain. In the chair form, substituents occupy either axial (up or down, along the imaginary axis of the ring) or equatorial (around the equator of the ring) positions.
**Key Points to Consider:**
1. **Axial and Equatorial Positions:**
- Axial positions are more sterically hindered and less stable compared to equatorial positions.
- Larger groups prefer the equatorial position due to reduced steric interactions with other axial hydrogen atoms.
2. **Substituent Placement:**
- The given structure shows an axial and equatorial designation for certain substituents.
- To ensure the most stable conformation, larger substituents should be placed in the equatorial position where there is less steric hindrance.
### How to Draw the Most Stable Conformation:
1. **Identify Substituents:**
- Determine the positions of all substituents on the cyclohexane ring.
- For this example, there is a mono-substituted methyl group shown on the cyclohexane.
2. **Substituent Size Consideration:**
- For a mono-substituted cyclohexane, the chair conformation should place this substituent in the equatorial position to minimize steric strain.
3. **Drawing Steps:**
- Draw the cyclohexane ring in its chair conformation.
- Add the substituent (methyl group in this case) in the equatorial position.
- Adjust any remaining hydrogen atoms to occupy the appropriate axial or equatorial positions, ensuring maximum stability.
### Conclusion:
By drawing the cyclohexane in its most stable chair conformation with the given substituent, stability is maximized and steric hindrance minimized. This principle is fundamental in organic chemistry for understanding the behavior of cyclic molecules.
**Note:**
This explanation aids in visualizing and understanding
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw (1R,3S)-1,3-dimethylcyclohexane, in its minimum energy conformation. Then draw the Newman Projection for this structure with the correct stereochemistry and in its minimum energy state.arrow_forwardDraw as many resonance structures as you can for the following carbonation. Label all of the carbonation structures as 1,2,3 (primary,secondary,tertiary) and circle the most stable conformation in terms of the carbonation.arrow_forwardStarting from the structure below (sighting down the indicated bond). rotate the back carbon to provide an eclipsed conformer of the structure. Ph H H HI!!! ++||||4 CH₂CH3 I I I I I I H Draw Newman Projection I I Iarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY