
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
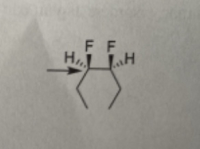
Draw the most stable Newman projection for the following
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 8: Identify each of the following disubstituted cyclohexane rings as either cis or trans. Draw the chair flip of each ring. Br CH3 Ici CH3 Br H3C- CH3 please explainarrow_forwardDraw each of the following. (a) Draw a Newman Projection of the following molecule, looking down the C3-C4 bond. Draw C3 in the front. CI Br H (b) Draw a Newman Projection of the most stable and the least stable conformation of the following molecule. Label each H H. CI H CI Harrow_forward1) Draw 2 sets of molecules. The first set should be constitutional isomers. The second set should not be constitutional isomers. Explain why the first set of isomers is constitutional and why the second set is not.arrow_forward
- Starting from the structure below (sighting down the indicated bond). rotate the back carbon to provide an eclipsed conformer of the structure. Ph H H HI!!! ++||||4 CH₂CH3 I I I I I I H Draw Newman Projection I I Iarrow_forward10) Draw a Newman Projection along the C3-C4 for the molecule shown below. (C3 should be in front.) C3 C4 HO OHarrow_forwardDraw the most stable Newman projection for the following alkanes looking down the C-C bond pointed to by the arrows.arrow_forward
- Explain why pleasearrow_forwarddraw all hydrogen atoms explicitly 2,4-diboguso-1-propylbenzene see Bg on attached special periodic tablearrow_forward, draw its and then in the 2. For (1R, 2S, 4R)-4-tert-buyl-1-ethyl-2-methylcyclohexane: draw its correct skeletal structure most stable conformer first showing the Hydrogens that are directly attached to the ring last stusture omit the hydrogens that are directly connected to the ring and draw the correct positions of the three alkyl groups in skeletal representation. Draw all bonds with the same length! 5. draw the correct skeletal structure here showing stereochemistry on this chair draw the most stable conformer including the H's directly attached to the ring on this chair draw only the alkyl groups, all bonds same lenghtarrow_forward
- Please don't provide handwriting solutionarrow_forwardProblem: Perform a conformational analysis 2-iodo-2-methylbutane, looking down the C2—C3 bond. Pay attention to the relative energies of the various conformations, but do not concern yourself with the actual energy values. Pleae draw newman projections.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY