
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Draw the most stable and least stable Newman Projections of 2,3-dimethylbutane by
viewing along the C2-C3 bond.
Expert Solution
arrow_forward
Step 1
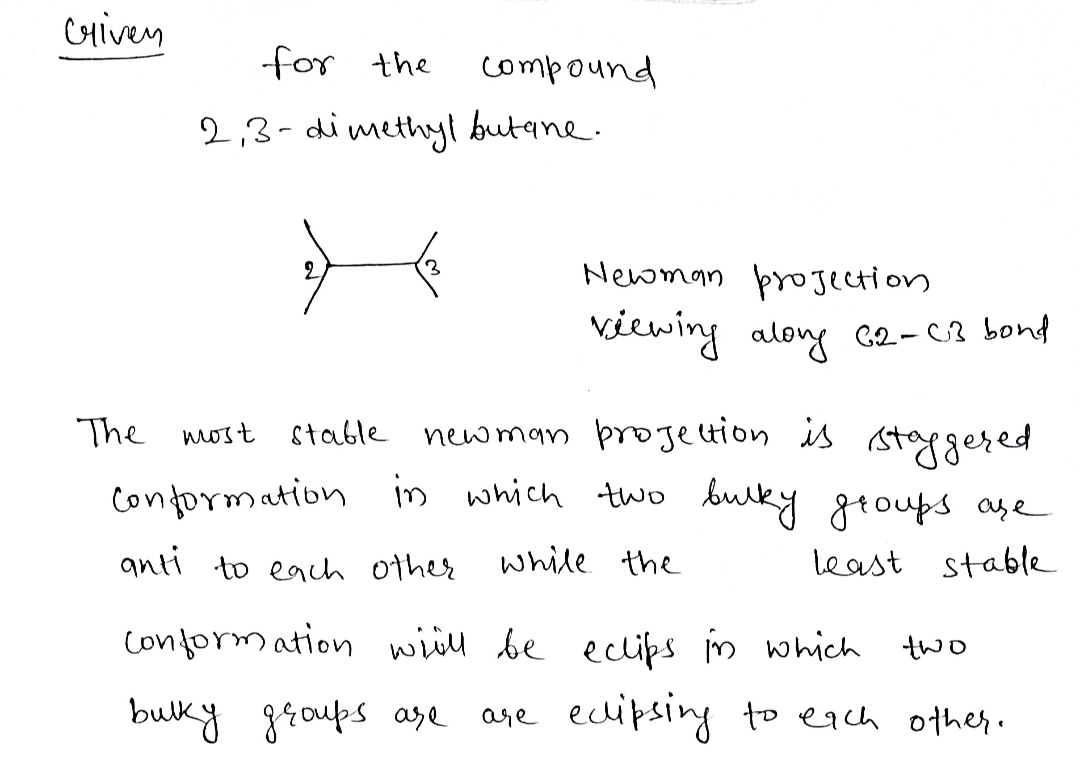
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) Re-draw the following molecule with the CH2CH3 group on the ladder 1. H. H2 CF H3C H2 CH2 H;C Is the molecule in part (a) the same molecule or the enantiomer of the following. Circle your answer Explain your answer CH3 CH2 H,C SAME MOLECULE CH3 ENANTIOMER H2arrow_forwardConsider (2R,3R)-2,3-dioidobutane: (a)Draw a Fischer Projection of this structure with C1 on top and C4 on bottom. (b)Draw a Newman Projection of of the meso compound of 2,3-dibromobutane looking down the C2-C3 bondwith C2 in front, and label your asymmetric carbons as S or R.arrow_forwardWhich Newman projection corresponds to the conformation of this compound that can undergo E2 elimination? Et CI E2 ? H tBu tBu tBu H H Me Et. H Me H CI tBu CI H CI H CI H Me Et Me Et A B Darrow_forward
- Draw two chair conformer of (1S,2R,4R)-4-chloro-2-methylcyclohexan-1-ol that would result from a chair flip. Include all groups of the three stereocenters, but no other hydrogens. Which conformer is favored at equilibrium?arrow_forwardAt room temperature, the 'H NMR spectrum of all-cis-1,2,3,4,5,6-cyclohexanehexacarboxylic acid exhibits two signals for the H atoms directly bonded to the ring. Explain why. Hint: Draw its chair conformation explicitly. CO,H HO,C .CO2H HO,C* *CO2H CO,Harrow_forwardYour answer is incorrect. Try again. Which staggered Newman projection(s), looking down the C-2-C-3 bond (C-2 in front and C-3 in back), illustrates the following boxed compound? H₂C CH₂CH3 CI CH3 I H₂C H I, II and III II and V I and III CH₂CH3 CH3 IV H₂C IV only None of these choices. II CH₂CH3 CH3 CH3 H H * H H V CH 3 H CH₂CH3 III CH(CH3)CHCICH 3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY