
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Draw the mechanism of the transformation from 3 to 4 .
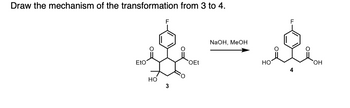
Transcribed Image Text:Draw the mechanism of the transformation from 3 to 4.
F
EtO
HO
3
OEt
NaOH, MeOH
HO
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 18.04a5 Add curved arrow(s) to draw the final step of the mechanism. H₂O *-* CH3 H3C H3C H₂C H N CH3 Edit Drawing H₂C NO₂ CH3 CH3arrow_forwardDraw the structure of all products of the mechanism below. ✓+ H₂C-C=C-C-OH H + Click and drag to start drawing a structure.arrow_forwardCircle the best nucleophile in ammonia: NaCl, NaOH, NaCH3, Nal Circle the best nucleophile in acetone: NaCl, NaOH, NaCH3, Nalarrow_forward
- Add curved arrow(s) to draw step 3 of the mechanism. Modify the given drawing of the product as needed to show the intermediate that is formed in this step. CH, H,O ÇH, CH, CH, H,C H,C CH, CH, of TY N Z +1 +tarrow_forwardDraw the structure of all products of the mechanism below. c* + H H H H H Click and drag to start drawing a structure. но H :0:arrow_forwardDraw curved arrows to depict the second step of the given mechanism. H d H Edit Drawing H H 8arrow_forward
- Draw the curved arrows for the following mechanisms. Describe what is hap step (mechanism patterns). (Take it one step at a time and note the differences). :0: R CI: H-O-H :Ö:0 :0: :CI: :CI: H2O OH OH2 Br Br H-Br O-H H-8-H :0: OH Br 1 Br H2O H₂Oarrow_forward10. a) In the box at the left, indicate whether the reaction takes place through an SN2, SN1, E2, or E1 mechanism. b) Draw the mechanism and predict the product, including stereochemistry. OH H3O* heat CI NaCN DMF KOt-Bu Br HOH HCIarrow_forwardWrite the correct mechanism and major product for a), and indicate the reagent needed for b) to happen.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY