
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Please show in line-angle (skeletal) form.
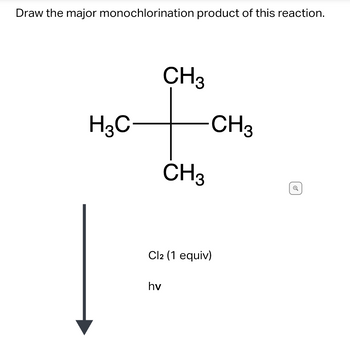
Transcribed Image Text:Draw the major monochlorination product of this reaction.
H3C
CH3
hv
CH3
CH3
Cl2 (1 equiv)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Induction can be simplified as the donation or withdrawing of electrons due to long range electronegative effects through sigma bonds True or False?arrow_forwardThe face indicated by the arrow in the molecule below is the: A) Re face B) Si face C) R face D) S face H₂C H3C II. O H CH3 CH 3arrow_forwardG.(H,C),CHCHCHCH(CH;)CCH Lewis Dot Structure Valence Electron Count Perspective/ 3D Line Bond/Skeletalarrow_forward
- H H TI H (1) H C H H H (3) H C H H The ideal value for bond angle (1) is (Choose one) ▼ and the ideal value for bond angle (3) is (Choose one) degrees, the value for bond angle (2) is (Choose one) degrees. degrees,arrow_forwardDraw the second best resonance structurearrow_forwardPlease don't provide handwritten solution ....arrow_forward
- 1X B - с emaining Time: 42 minutes, 34 seconds. westion Completion Status: Moving to the next question prevents changes to this answer. estion 2 AC 10¹HHIHIS || https://bbhosted.cuny.edu/webapps/assessment/take/take.jsp?course_assessment_id=... A linear, bond angle is 180° O trigonal pyramidal; bond angle is 109.5⁰ Obent; bond angle is about 1200 bent; bond angle is about 109.50 trigonal planar; bond angle is 1200 7 points Save Am In the hypothetical molecule SeCl2, the central atom Se has two lone pairs of electrons in addition to the two bond pairs in the Se-Cl bonds. What is the shape of this molecule? (Hint: draw it!) O Moving to the next question prevents changes to this answer. aff and on 2 W S alt # 3 E C ▬▬ ES $ 4 R F to go 96 5 T G B 14- d- 6 Y H & N 7 U H M HAN 8 1| 15 | 154 1|a1|=| FSA FSA 9 1 JL K O hopp! L O to V B Ⓒ 6 alt P : - ? 99+ ( C ** insert a 1 MC MC 1 + ctri + } pause prt sc ] 1 4 Question 2 c Question 2 of 17 1 ( Ⓒ D backspace enter T shift 8 10/2-arrow_forwardIn 3-5 sentences, describe the differences between molecules that are polar and those that are non polar.arrow_forwardDraw the Lewis structure and geometry shape for XeF2 Don't forget to show the number of available valance electrons. You don't need to type anything here. Edit View Insert Format Tools Table 12pt v Paragraph v BIU A e T? v : IL Proctorio is 画arrow_forward
- ← Canvas at ECU X Course Home X P Acceptable uni X Qh3c-h bond dis X с ✰ openvellum.ecollege.com/course.html?courseld=17588559&OpenVellumHMAC=84132a2235da8b06af3b7950d59cf661#10001 Course Home Mastering Chemistry Course Home My Courses Syllabus Scores eText Document Sharing User Settings Course Tools ● Dashboard xb My Math Solve X 56°F Sunny Chem 1120: Intro to Gen Chem for the Allied Health Sciences. 0 … Help x ⠀ 5:01 PM 11/13/2022arrow_forwardCould you like to help me, pleasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY