
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Give detailed mechanism Solution with explanation needed..don't give Handwritten answer...give answer both sub parts if you not then don't give answer
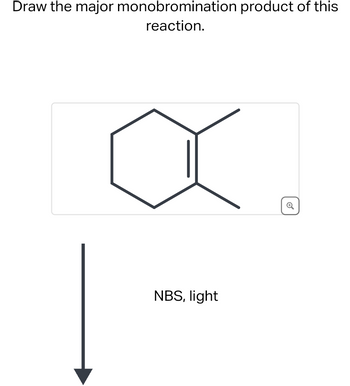
Transcribed Image Text:Draw the major monobromination product of this
reaction.
NBS, light
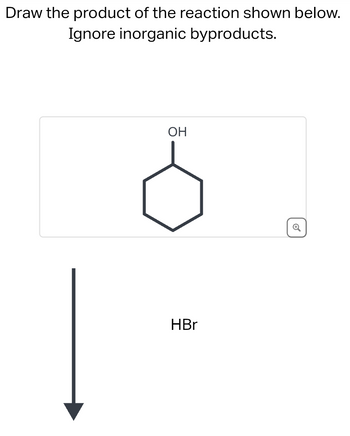
Transcribed Image Text:Draw the product of the reaction shown below.
Ignore inorganic byproducts.
OH
HBr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The representation of the potential of an indicator electrode against the logarithm of the analyte activity is...(1). a straight line for all analyte concentrations. (2). a curve with zero slope for any concentration value below a certain limit. (3). an exponential curve. (4). a logarithmic curve.arrow_forwardcable below you will find three descriptions of sclentists engaging In activitles that are part of the sclentifi description missing word? Carl records In his lab notebook the powder In a test tube, the powder melts into a silvery liquld that welghs less than the original powder. that when he heats a red Antolne develops a idea that most substances will chemnically combine with an invisible gas in the atmosphere he calls oxygen. Antoine uses hls ideas to explain burning as the comblnation of a substance with oxygen, and reduction (e.g. durlng che formation of iron from iron ore) as the splitting of a substance from a chemical combination with oxygen. for chemical reactions based around the ohn analyzes all the known compounds of carbon and oxygen. He finds nd writes down a that says the ratio of masses of carbon that combine with 100 g of oxygen in these compounds are always in whole umber ratios (e.g. 2:1 or 3:1).arrow_forwardWhat is Fertilizer Production in a chemical Industrial Process? A) Explain what is produced, B) What chemical reaction(s) are involved. C) Why this process/product is important? D) Finally show how an understanding of limiting reagents is important in optimizing this process. E) provide at least Iwo reference URL legitimate sources Note; Please explain clearly, With a full sentence free of plagiarism.arrow_forward
- What will happen to MP (effect and range) if one uses a melting point tube with too large a diameter?arrow_forwardin text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forwardgive me mechanism, please explain each steparrow_forward
- The addition of concentrated nitric acid to each standard solution... Select all that are True. changes the potential of the reference electrode. results in a low pH, which is required for this experiment. results in a wet acid digestion to ensure sample dissolution. results in a relatively constant ionic strength across the standard solutions. results in the required amount of excess nitrate ion. Tries 3/6 Previous Triesarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardCan it please be explain just like in the uploadarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY