
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
G.260.
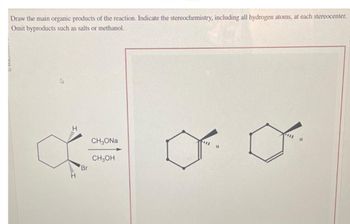
Transcribed Image Text:Draw the main organic products of the reaction. Indicate the stereochemistry, including all hydrogen atoms, at each stereocenter.
Omit byproducts such as salts or methanol.
4
Br
CH₂ONa
CH₂OH
H
HIE
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. For, 4 CuzN (s) + 3 02 (g) → 6 Cu20 (s) + 2 N2 (g) (a) Find the mass of Cu,0 formed by using 6.0 grams of copper (1) nitride, Cu;N. Given amount Answer Work (by dimensional analysis) and units with units (b) Find the mass of Cu,0 formed by using 1.0 g of oxygen, 0,. Given amount Answer Work (by dimensional analysis) and units with units (c) Which reactant is the limiting reactant? (Cu3N or 02)arrow_forwardSoda ash (sodium carbonate) is widely used in the manufacture of glass. Prior to the environmental movement much of it was produced by the following reaction. CaCO3 + 2 NaCl → Na2CO3 + CaCl2 Unfortunately, the byproduct calcium chloride is of little use and was dumped into rivers, creating a pollution problem. As a result of the environmental movement, all of these plants closed. Assume that 125g of calcium carbonate (100.09 g/mol) and 125 g of sodium chloride (58.44 g/mol) are allowed to react. Determine how many grams of useful sodium carbonate (105.99 g/mol) will be produced. How many grams of useless calcium chloride (110.98 g/mol) will be produced? You should also determine how many grams of excess reactants are left (indicate which one is the limiting reactant)arrow_forwardThe great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. х10 2. Suppose 14.0 mL dioxygen gas are produced by reaction, at a temperature of 110.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits.arrow_forward
- Consider a small bird of mass 30 g. What is the minimum mass of glucose that it must consume (and burn) to fly up to a branch 10 m above the ground?arrow_forwardA sampe compound of xenon and fluroine conatains moleucles of a single type; XeFn, where n is a whoel number. If 9.03 x1020 of these XeFn ole cules have a mass of 0.311g, hwat is the value of n?arrow_forwardA 19.51 g sample of impure methylamine, which contains 72.58% (by mass) of CH;NH: , is reacted with 30.81 g of pure oxygen gas: 2.1 4CH, NH,(2) + 90,(8) - 4C0,(8) + 10H,0(?) + 2N;(8) 2.1.1 What is the percentage yield of this reaction if 5.54 g of nitrogen gas is collected? 2.1.2 In another experiment, this impure methylamine was used as follows: • An unknown mass of the impure compound is dissolved in enough water to make 500.0 m of solution. • 20 ml of this solution was transferred by pipette to a clean 250 ml volumetric flask and made up to the mark. • The molarity of the CH;NH; in the final solution was determined to be 0.103 M. Determine the mass of CH;NH; present in the original amount of impure compound used make this solution.arrow_forward
- use 17.89 mL (experimental volume) and 0.00074044 mol H2 (theoretical moles of hydrogen gas) to calculate the value of R. Include correct unitsarrow_forward14. The fertilizer ammonium sulfate [(NH,),SO4] is prepared by the reaction between ammonia (NH3) and sulfuric acid: 2NH;(g) + H,SO,(aq) --->(NH,),SO,(aq) How many kilograms of NHz are needed to produce 1.00 x 10° kg of (NH,),SO,?arrow_forwardThe metal widely used in our civilization is iron (Fe) and it is obtained industrially by the reduction of iron oxide (Fe,0,) with carbon at higher temperatures (over 1000 °C). The equation for the reaction is as follows, Fe,0;(s) + C(s) –Fe(s) + CO2(g) Using the above equation, a. What is the number of moles of Fe? (ensure 3 decimal places for e.g. 0.300) mols b. How much of the product (Fe) can be produced from 10.0g of Fe,O;?(ensure 2 decimal places for e.g. 0.30) c. What is the number of moles for carbon?(ensure only 4 decimal places) (ensure 4 decimal places for e.g. 0.3003) mols boarrow_forward
- The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 2. Suppose 53.0mL of dioxygen gas are produced by this reaction, at a temperature of 50.0°C and pressure of exactly 1atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits.arrow_forward1:39 1 Send a chat Enter your answer in the provided box. Hydrogen fluoride is used in the manufacture of Freons (which destroy ozone in the stratosphere) and in the production of aluminum metal. It is prepared by the reaction CaF2 + H2SO4 → CASO4 + 2HF In one process, 5.75 kg of CaF, is treated with an excess of H,SO, and yields 2.45 kg of HF. Calculate the percent yield of HF. % yieldarrow_forwardA compound contains only Mn and F. The ratio of Mn and F is 2.54 moles of F per 1 mole of Mn. If the empirical formula is expressed at Mnfx, what is the numerial value of x?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY