
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I don't get it
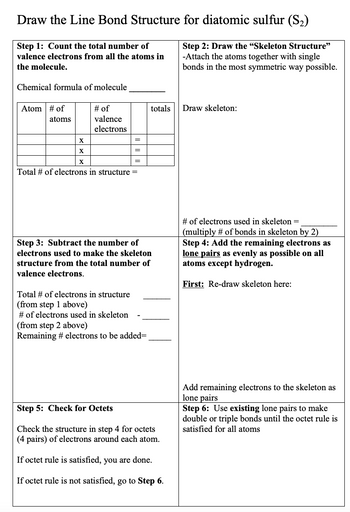
Transcribed Image Text:Draw the Line Bond Structure for diatomic sulfur (S₂)
Step 1: Count the total number of
valence electrons from all the atoms in
the molecule.
Step 2: Draw the "Skeleton Structure"
-Attach the atoms together with single
bonds in the most symmetric way possible.
Chemical formula of molecule
Atom # of
atoms
X
X
X
# of
valence
electrons
=
=
=
Total # of electrons in structure =
totals
Step 3: Subtract the number of
electrons used to make the skeleton
structure from the total number of
valence electrons.
Total # of electrons in structure
(from step 1 above)
# of electrons used in skeleton
(from step 2 above)
Remaining # electrons to be added=
Step 5: Check for Octets
Check the structure in step 4 for octets
(4 pairs) of electrons around each atom.
If octet rule is satisfied, you are done.
If octet rule is not satisfied, go to Step 6.
Draw skeleton:
# of electrons used in skeleton =
(multiply # of bonds in skeleton by 2)
Step 4: Add the remaining electrons as
lone pairs as evenly as possible on all
atoms except hydrogen.
First: Re-draw skeleton here:
Add remaining electrons to the skeleton as
lone pairs
Step 6: Use existing lone pairs to make
double or triple bonds until the octet rule is
satisfied for all atoms
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Shop Origami Owl O Inbox Outlook W... Sugarpill Cosmet.. 3 of Constants I Periodic Part A Li2 CO3(s)- Express your answer as a chemical equation. Identify all of the phases in your answer. ΑΣφ ? Submit Request Answer Part B CDCO3 (s) Express your answer as a chemical equation. Identify all of the phases in your answer. ? AEp -ΑΣΦ P Pearson MacBook Air tarrow_forwardEto новски знаеха شت HOarrow_forwardFill in the missing information. ОН CH3 H+ НО Br- ОН m-CPBA ОН Br H2 Pd/C 1. NaBH4 2. H2O+ Іarrow_forward
- Icalate Hi BeHot)+6el2( +2Bels() +oHel(②) using Hess Law Bel310+3H2O→H2B/23(g) +3=-122.5K) B2H6(+6H2O(P)→2HB+6H2(火) →トニ-4734人) Male B₂ HO H2(+244145) Siven +3HP Im Belzarrow_forwardна о кмпоч, Лон Изоб is the Вгг ха 1. ВНЗ 2. H2O2, ное вс + X ник Br пре CIarrow_forwardI have attached a picture of the problems I'm struggling with.arrow_forward
- Need help with a-k! Pleasearrow_forwardDetermine the slope of the line. 3. 2+ 1+ 1 4 6. -1+ X-axis -2. -34 -4+ O -2 O 2 0.5 O 0.5 N. y-axis -1arrow_forward9. Determine whether the following drugs comply with Lipinski's rule. Also investigate the "alternative criteria" (rotatable bonds, polar surface area) for both drugs. OH N(CH3)2 HO. OH OH OH O Tetracycline (antibiotic) P=0.04 OH CONH₂ OH N. Naloxone (morphine agonist) P=83arrow_forward
- Which is not a type of molecular motion? Rotation Translation O Vibration O Shimmyarrow_forward46) CH3 (CHe), E cH, + NH, EN HNH, 47) CH3 (CH2)4 ECHa + cHz eHq NH 48) (CH)C=0 2 CHE CHR 아 4인 50) cH2 Zn /Hce si) Li Al Hot, cHz 5) CH3 ce (aea) + 시H37 ce Cexces) + 사3-7 53) NH3 (ayceas) + CHs e ->arrow_forwardanswer with states pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY