
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
! ( given ans with explanation and mechanism )
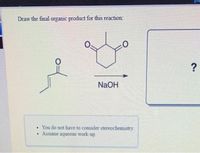
Transcribed Image Text:Draw the final organic product for this reaction:
NaOH
• You do not have to consider stereochemistry.
Assume aqueous work-up.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Help! Please explain in detail. Thank you! Consider the following overall reaction and proposed mechanism. Evaluate whether each step of the mechanism is reasonable. For each step that is not reasonable, explain why.arrow_forwardOrganic Chemistry Problem. Please help. Please provide neatly written mechanism using arrows to show electron flow and all intermediates. Thank you.arrow_forward:$:$;);$):):$$:$;$;$arrow_forward
- Give detailed mechanism Solution with explanation needed..avoid handwritten Solutionarrow_forwardGive detailed mechanism Solution with explanation needed of each steps..don't give Handwritten answerarrow_forwardGive mechanism for the following: OH .... * Me Me THF Li/NH3 (51%) ... I *** m Me " Me OEtarrow_forward
- Organic Chemistry Problem. Please help. Please provide neatly written mechanism using arrows to show electron flow and all intermediates. Thank you.arrow_forwardGive detailed mechanism Solution with explanation needed..don't give Handwritten answer...arrow_forwardWhat woud them mechanism be to go from cyclohexanol to methylcyclohexanol?arrow_forward
- Organic Chemistry Problem. Please help. Please provide neatly written mechanism using arrows to show electron flow and all intermediates. Thank you. (Please don't use hend raiting)arrow_forwardOrganic Chemistry Problem. Please help. Please provide neatly written mechanism using arrows to show electron flow and all intermediates. Thank you.arrow_forwardDetermine dissociation constant of a WA: given pH of the half-neutralized acid solution is pH=5.1: What is pKa and Ka? What is the unknown weak acid? Also given: NaHSO4 Ka = 1.2 x 10-2 KHC8H4O4 (KHP) Ka = 8.7 x 10-6 for context, earlier part of experiment was the below (may not be needed for problem) Record the pH and the color observed with bromcresol green for each of the 0.1 M solutions that were tested. NaCl Na2CO3 NaC2H3O2 NaHSO4 pH 7 11 8.0 1.9arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY