
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
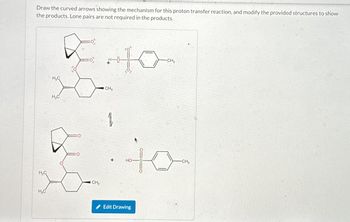
Transcribed Image Text:Draw the curved arrows showing the mechanism for this proton transfer reaction, and modify the provided structures to show
the products. Lone pairs are not required in the products.
H₂C
H₂C
H₂C
H₂C
CH3
+10-
CH3
+
HO
Edit Drawing
-CH3
10
-CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- show the major product/s of the given reactionarrow_forwardWrite an equation for the proton transfer reaction that occurs when the following base reacts with water. Draw curved arrows that show a mechanism for the proton transfer, and modify the given structures to draw the resulting products. \ 7 11 TYZ NV + OCX D H₂C H₂C- डे H.C Di H₂C H-01H O I N O P S F LL CI Br I L 141arrow_forwardSelect the major product of the chemical reaction below. Br EtOH EtO C OA OEt A B C OEt D E F LLarrow_forward
- Draw the product when A (TOP) and B (BOTTOM) react together: (Ignore the SOCl2)arrow_forwardProvide an arrow pushing mechanism for the following reaction. Include protons and each step clearly indicated. Br H. D H3C Hz H3C CH3arrow_forwardComplete the curved arrow mechanism of the following double elimination reaction when 1,2-dibromopropane is treated with two equivalents of sodium amide and heated in mineral oil. a) Use three curved arrows to show the elimination of the first hydrogen bromide. HN: H 19 H : Br H ↑ b) Use three curved arrows to show the elimination of the second hydrogen bromide. : Br | H H : B H Harrow_forward
- OH H xs CrO3 H₂O acetone Modify the structure of the starting material to draw the major organic product. Use the single bond tool to interconvert between double and single bonds. Edit Drawing ? OHarrow_forwardWhich of the four options below is a correct product to the following reaction H₂NOC H₂NOC H₂C HN 20 Ho HN CO₂H H₂NOC zo HN NaOH/H₂O CFCOH H₂O+arrow_forwardAdd curve arrows to draw step 4 of the mechanism.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY