
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
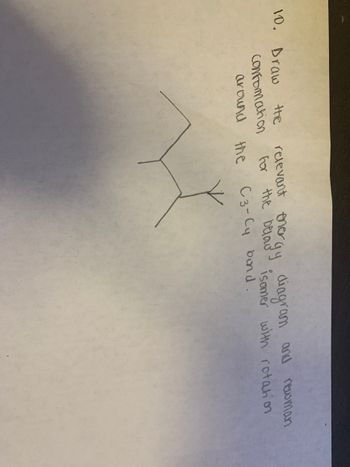
Transcribed Image Text:**Question 10:**
Draw the relevant energy diagram and Newman conformation for the butanyl isomer with rotation around the C3-C4 bond.
**Explanation:**
The image shows a simple molecular diagram indicating the C3-C4 bond along with what seems to be a skeletal formula. The task involves visualizing different rotations around this bond to identify conformations like staggered, eclipsed, or gauche, and then drawing an energy diagram demonstrating the relative energy differences between these conformations. The Newman projection is a valuable tool for visualizing these rotations and their impact on molecular energy and stability.
Expert Solution
arrow_forward
Step 1
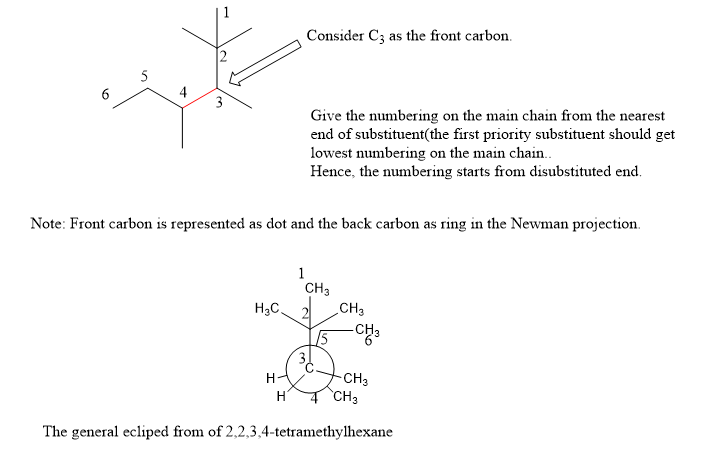
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following two pairs of molecules, (1) Draw out the chair conformation for each molecule, flip the ring if it is possible. (2) Compare both molecules to circle out which one is more stable. (3) Identify their relationship as: constitutional isomer, conformational isomer, stereoisomer or identical. (4) Find all the chiral center on each molecule and label them. VS. ****arrow_forwardIt is easy to imagine a cyclohexane as a flat hexagon and a lot of the time we draw it that way. Looking at 1,3,5-triethylcyclohexane we cannot tell the stability of the molecule from looking at the flat 2D drawing. Explain why we need to look at the 3D configuration and what conformation (axial,equatorial) would each of the three ethyl groups be in for the most stable configuration.arrow_forward• Question 3: a) Draw the three staggered and three eclipsed conformations that result from rotation around the designated bond using Newman projections. b) Label the most stable and least stable conformation. rotation here H CH3 C CH₂CH3 CH3arrow_forward
- What is the approximate dihedral angle between the two chlorine atoms in the diequatorial conformation of trans-1,2-dichlorocyclohexane? **answer is NOT 180 degrees. Explain with drawing of the structure. a) 180 degrees b) 120 degrees c) 60 degrees d) 0 degreesarrow_forwardGive me answer for chemistry organic chemistryarrow_forwardQUESTION 3 (a) Draw the two conformations of ethane which has an energy minimum and energy maximum using the Newman projection. Explain why one conformation is lower in energy than the other.arrow_forward
- Draw Newman projections for the anti, eclipsed, gauche and fully eclipsed conformations of Butane. Indicate their relative stabilities and the reasons for your ranking-need to discuss the stresses involved in each case. Using suitable specific conformers of specific structures as examples, explain the difference between (a) angle strain (b) torsional strain and (c) steric strain. Need to draw the confomers and structures 4) Draw the most stable conformers using chair structures of the following: (c) cis-1,2-Dibromocyclohexane (d) trans-1,2-Dibromocyclohexane (e) cis-Decalin (f) trans-Decalin . Draw the most stable conformers of trans-1-Bromo-4-methylcyclohexane and cis-1-Bromo-4-methylcyclohexane. Which is more stable? Explain the reason for your answer. Need to include a discussion of 1,3-Diaxial interactionarrow_forwardConsider rotation around the carbon–carbon bond in 1,2-dichloroethane (ClCH2CH2Cl).Using Newman projections, draw all of the staggered and eclipsed conformations thatresult from rotation around this bond.Graph energy versus dihedral angle for rotation around this bond.arrow_forwardI don't understand how to tell which one goes on which side. When I looked at the answer key to check my answer, I had H/Br and H/NH2 on the wrong sidesarrow_forward
- 1. Does chlorophyll a or chlorophyll b have a greater number of “conjugated” double bonds, or do they have the same number? Draw the structure of chlorophyll b and using a colored highlighter, indicate which of the double bonds are “conjugated”. 2. What is the main functional group in THF (tetrahydrofuran)? Is THF more polar or less polar than IPA (also called: isopropyl alcohol, isopropanol, or 2-propanol)? 3.If you mistakenly used hexanes-THF (2:1) for eluting the carotene pigments from your chromatography column, will they elute faster or slower than if you had correctly used hexanes-THF (10:1)? Explainarrow_forwardUse the conformation below to answer the following questions. F. a) Write the IUPAC substitutive name of this compound b) Is this the most stable conformation of this molecule? You will only get one submission for this part of the question - choose wisely. ---Select--- îarrow_forward[Review Topics] [References] Draw two constitutional isomers that share the molecular formula C3H6. Your structures will have the same molecular formula but will have different connectivities.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning