
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
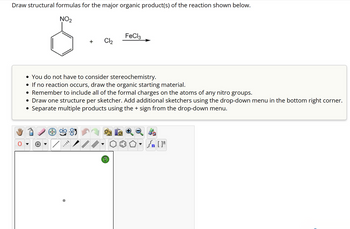
Transcribed Image Text:### Reaction Instructions for Drawing Structural Formulas
**Objective:**
Draw structural formulas for the major organic product(s) of the reaction shown below.
#### Reaction Details:
- **Starting Material:** Nitrobenzene (C₆H₄NO₂)
- **Reagents:** Chlorine (Cl₂), Ferric chloride (FeCl₃) catalyst
#### Guidelines:
- **Stereochemistry:** You do not have to consider stereochemistry for this reaction.
- **Non-reactive Scenario:** If no reaction occurs, simply redraw the starting material (nitrobenzene).
- **Formal Charges:** Ensure all formal charges are correctly included on the atoms of any nitro groups present.
- **Drawing Tips:**
- Draw one structure per sketcher.
- Use the drop-down menu in the bottom right corner to add additional sketchers if needed.
- To depict multiple products, separate them using the '+' sign found in the drop-down menu.
#### Diagram Editor:
- **Interface Overview:**
- The diagram editor includes various tools for drawing and modifying chemical structures.
- Utilize the provided templates for ring structures or functional groups as necessary.
- Access bond and atom types from the toolbar for precise drawing.
This exercise is meant to enhance understanding of electrophilic aromatic substitution in benzene derivatives, specifically focusing on chlorination in the presence of a catalyst.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the product(s) of the following reactions. HO H₂C C-CEC-CH3 H H₂ Lindlar catalyst • Consider E/Z stereochemistry of alkenes. • If no reaction occurs, draw the organic starting material. TAYY • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. ? [Review Topics] ChemDoodleⓇ [References] Begarrow_forwardDraw the organic product(s) of the following reaction. O OCH3 aqueous HCI • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. ● ChemDoodleⓇ Sn [Farrow_forwarddraw the structure(s) of the major product(s) of the followingarrow_forward
- Draw structural formulas for the major organic product(s) of the reaction shown below. CH₂CH3 + 8 Br2 • You do not have to consider stereochemistry. • If no reaction occurs, draw the organic starting material. • Remember to include all of the formal charges on the atoms of any nitro groups. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. FeBr3 ? [ ] در ? O. Sn [F Previous Nextarrow_forwardDraw structural formulas for the major organic product(s) of the reaction shown below. CI • • • FeBr3 Br2 You do not have to consider stereochemistry. If no reaction occurs, draw the organic starting material. Remember to include all of the formal charges on the atoms of any nitro groups. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu. ? n [] ChemDoodlearrow_forwardH3C H H2ö Product(s) ČH3 Predict the product(s) of this reaction by interpreting the flow of electrons as indicated by the curved arrows. • You do not have to consider stereochemistry. • You should include all products. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu.arrow_forward
- Draw the organic product(s) you would expect from the following reaction. Assume products derive from the most stable carbocation intermediate(s). se + HCI (1 mole) You do not have to consider stereochemistry. • If there is more than one major product possible, draw all of them. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the+sign from the drop-down menu.arrow_forwardQuestion 7arrow_forwardDraw structural formulas for the major organic product(s) of the reaction shown below. CI • • • H2SO4 + HNO3 You do not have to consider stereochemistry. If no reaction occurs, draw the organic starting material. Remember to include all of the formal charges on the atoms of any nitro groups. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu. ▼n √n [ ? ChemDoodlearrow_forward
- Draw the structure of the major organic product(s) of the reaction. CH3 S You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. /// CI ? 1.2 MgBr, ether 2. H₂0+ | [ ]# Previous Nextarrow_forward[Review Topics] [Refer Draw structural formulas for the major organic product(s) of the reaction s CN AICI3 + (CH3)2CHCI • • • . . You do not have to consider stereochemistry. If no reaction occurs, draw the organic starting material. Remember to include all of the formal charges on the atoms of any Draw one structure per sketcher. Add additional sketchers using th corner. Separate multiple products using the + sign from the drop-down m 26224 N▾ ? [ ChemDoodle removearrow_forwardDraw the structure of the major organic product(s) of the reaction. olso OH • You do not have to consider stereochemistry. • All carboxyl and amino groups should be drawn in the neutral form. • If no reaction occurs, draw the organic starting material. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY