
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
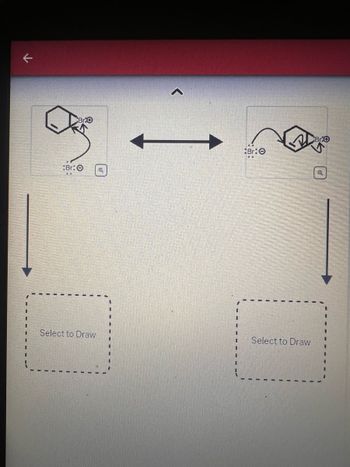
Curved arrows are used to illustrate the flow of electrons . Use the reaction conditions provided and follow the curved arrows to draw the products of the following reaction . Predict the reactants based on the intermediates provided . Include all lone pairs and charges as appropriate .
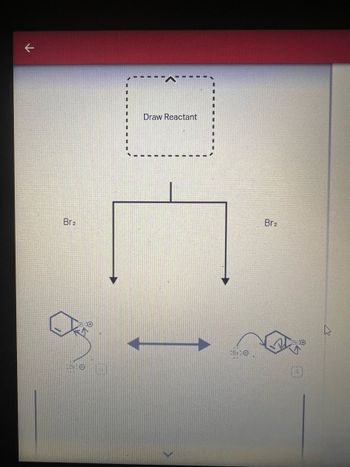
Transcribed Image Text:Draw Reactant
Br2
Br2
Bite
BIO
Transcribed Image Text:Br
Br:O
a
Br:O
Select to Draw
Select to Draw
(0)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- The following reaction is one step in the pathway to remove ammonia from the body. Curved arrows are used to illustrate the flow of electrons. Using the starting structure, draw the curved electron-pushing arrows for the reaction. Be sure to account for all bond-breaking and bond-making steps. Then draw the missing product of this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts, counterions, and solvents. 0:0 :0: HNH₂ 0:0 HH Base :O: R Select to Edit Arrowsarrow_forwardComplete the following acid-base reaction . Show all valence electrons on the interacting atoms and show by the use of curved arrows the flow of electrons in each reaction.arrow_forwardSee image belowarrow_forward
- See image belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting structure, draw the curved electron- pushing arrows for the following reaction or mechanistic steps. be sure to account for all bond-breaking and bond-making steps. then draw any missing organic intermediates or products for this reaction. include all lone pairs in the structures. ignore inorganic byproducts, counterions, and solvents.arrow_forwardComplete the equation for the following proton-transfer reaction by using curved arrows to show the flow of electron pairs and drawing the products of the reaction. • Draw all atoms, including hydrogen atoms. Apply formal charges where appropriate. Assign lone pairs and radical electrons where appropriate. • Use the "starting points" menu to revert to the original molecule(s) shown. • Draw the appropriate electron-flow arrows. • Omit + signs between structures. ● ● H-O: == starting points == ↑ TAYY H T H-C-H H-N-H T H در ? ChemDoodlearrow_forward
- Curved arrows are used to illustrate the flow of electrons. Follow the arrows and draw the missing intermediates and product formed in this reaction. Include all lone-pairs. Ignore any inorganic byproductsarrow_forwardKetones and aldehydes are hydrated under acidic or basic conditions. An example of the acid-catalyzed hydration is shown. Complete the first step of this mechanism. Include all lone pairs of electrons, curved arrows, and nonzero formal charges in your mechanism.arrow_forwardConsider the following reactants: H₂O Would substitution take place at a significant rate between these reactants? Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. If you said substitution would take place, draw all the major products in the upper drawing area below. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. If you said substitution would take place, also draw the complete mechanism for one of the major products in the lower drawing area below. If there is more than one major product, you may draw the mechanism for the production of any one of them. Note: be sure you show all the steps in your mechanism. A good check is to make sure the final product of your mechanism is a stable molecule that could be isolated and purified. Major Products: Click and drag to start drawing a structure. yes no X 9, X :0 Garrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediate and product of this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Use wedges and dashes to indicate stereochemistry. Ignore inorganic byproducts.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting structures, draw the curved electron-pushing arrows for a proton transfer reaction and draw the resulting product. Be sure to account for all bond-breaking and bond-making steps. O HH Base .0 H R HH HH •6•H :O: HH Select to Add Arrows Base Select to Draw Productarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY