
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
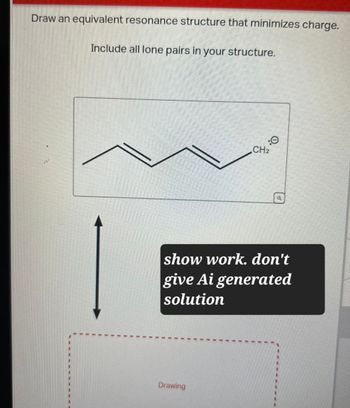
Transcribed Image Text:Draw an equivalent resonance structure that minimizes charge.
Include all lone pairs in your structure.
:0
CH2
show work. don't
give Ai generated
solution
Drawing
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- POST-LABORATORY QUESTIONS Due after Laboratory Experiment 1. Thiocyanate, SCN, has three possible structures a. Draw the resonance structures of thiocyanate, SCN- b. Assign the formal charge of each atom in each of the three resonance structures. On the basis of the formal charges and the electronegativities, identify the major resonance structure. с. Duour tho LOuris dot structure for each of the following molecules or ions. Determinearrow_forwardDraw a second resonance structure for each species in parts (a), (b), and (c). Draw two additional resonance structures for the ion in part (d).arrow_forwardS Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H H = HIN: H C. :0 H /\ H H Click and drag to start drawing a structure. ×arrow_forward
- Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. C H H C H :Ö: Click and drag to start drawing a structure.arrow_forwardConsider compounds A-D, which contain both a heteroatom and a double bond. ÖH A B a. For which compounds are no additional Lewis structures possible? b. When two or more Lewis structures can be drawn, draw all additional resonance structures.arrow_forward3. Consider the two resonance structures below (i and ii): N: :N N: ii A) Which structure obeys the octet rule? (circle one) i ii both neither B) On each structure, write the formal charge for each element. C) Which resonance structure do you anticipate being the major contributor? (Circle) i ii both neither :ö:arrow_forward
- Practice excercise 1arrow_forwardCan you please show step by step how to do this on Lewis structuress on a piece of paper.arrow_forwardDetermine the formal charge for the atom indicated in the structures below. ASSUME that the structures are drawn correctly and that you do not have to change them due to formal charge issues. The external atom in this structure. :O: :O! Ar 11 :0: The central atom in this structure. = :0: (1 Si = : 0: 2- 1. +1 2. +2 3. +3 4. 0 5. -3 6.-2 7. -1arrow_forward
- In the following resonance structure, 4 points what is the formal charge on the sulfur atom? * .. :S-C N: +1 O +2 Page 1 of 1arrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. H. +N=C H H H Cl: Click and drag to start drawing a structure. : ? g B S olo Ar B Karrow_forwardDetermine the formal charge for the atom indicated in the structures below. ASSUME that the structures are drawn correctly and that you do not have to change them due to formal charge issues. The external atom in this structure. H₂ _H ''H Ge A' Ī It 1. +1 2. +2 3. +3 4. 0 5. -3 6. -2 7. -1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY