
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Draw the alcohol starting material needed to produce the following alkyl chloride under the conditions shown. Use wedge and dash bonds to indicate stereochemistry where appropriate.
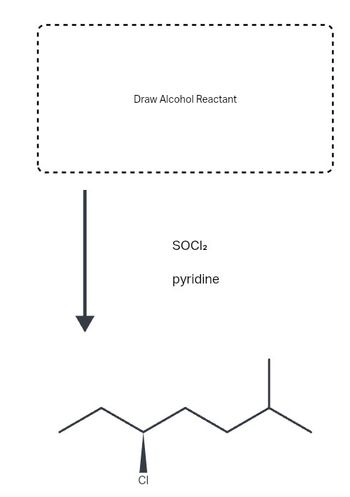
Transcribed Image Text:### Transcription of the Image for Educational Purposes
The image presents a chemical reaction schema involving an alcohol reactant. Here's a detailed description:
1. **Alcohol Reactant Box:**
- At the top of the image, there is a dashed rectangular box labeled "Draw Alcohol Reactant." This indicates that the specific alcohol reactant structure should be drawn here.
2. **Reaction Arrows and Conditions:**
- A downward arrow points from the box to the reaction conditions.
- The reaction involves **SOCl₂** (thionyl chloride) and **pyridine**. These are the reagents used for the transformation of the alcohol reactant.
3. **Product Structure:**
- The final structure at the bottom of the image is a chlorinated hydrocarbon.
- The product is displayed with a chlorine (Cl) atom attached to a carbon chain, signifying the replacement of the hydroxyl group (OH) of the alcohol with a chlorine atom.
This image illustrates the conversion of alcohols to alkyl chlorides using SOCl₂ and pyridine, a common method in organic chemistry for nucleophilic substitution reactions. The structure should be completed by drawing the appropriate alcohol reactant to showcase the process fully.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw an elimination reaction of 4-methylcyclohexanol using phosphoric acid including reaction conditions and minor products.arrow_forwardprovide the synthesis of Oleic acid from any straight chain alkanes containing 5 carbons or less. must make all reactants containing carbon from a straight chain alkane containing 5 carbons or less, but may utilize any reagents or solvents needed without making them.arrow_forwardstructure of 3-methylpentanal reacting with ethanol under acidic conditionsarrow_forward
- Provide stepwise synthesis show all reagents need and intermediates formed along the wayarrow_forwardGive the IUPAC name for the following compound. CH3-CH;-CH,-C-0-CH-CH, ethyl propanoate 4-hexanal ethyl propyl ether ethyl butanoate hexanoic acid « Previousarrow_forwardPlease explain step byarrow_forward
- Provide synthetic schemes for the followingarrow_forwardPlease do it neat and clean correctly on a plain white paper please thank you so mucharrow_forwardAcetal derivatives of aldehydes and ketones are prepared by an acid-catalyzed dehydration reaction with alcohols or diols. Using more pictures than words, draw a reaction and a mechanism that shows the formation of acetal.arrow_forward
- Using a series of reaction equations, outline how you would synthesize the following compounds from the reactants given. Show all reagents and necessary reaction conditions. You may use any reagents or solvents you cannot use starting materials other than those given. necessary, but a) 2,4-dimethyl-2-pentanol from 4-methylpentene and methyl bromide b) Heptanal from 1-heptene c) d) CH₂CH₂CH₂ CH2CH2CH2−O–C–CH3 from CH3 Ethylcyclohexane from CH3 -CH₂CH=CH₂ and tert-butyl alcohol aa CH₂Brarrow_forwarda) Write the mechanism and predict the product for the following reaction, including stereochemistry. CH;CH2OD is ethanol that is has deuterium in place of hydrogen on the hydroxy group. NaBH4 CH;CH2OD b) Show how you would use a Grignard reagent to convert the following ketone into the alcohol. This reaction requires two steps. ноarrow_forwardSynthesize 2-methylbutanoic acid from 2-methylbutene. Please provide all steps and reagents used.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY