
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please show every step for this problem, I am in need of help solving it ASAP!
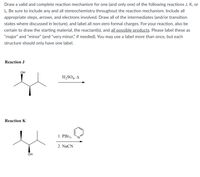
Transcribed Image Text:Draw a valid and complete reaction mechanism for one (and only one) of the following reactions J, K, or
L. Be sure to include any and all stereochemistry throughout the reaction mechanism. Include all
appropriate steps, arrows, and electrons involved. Draw all of the intermediates (and/or transition
states where discussed in lecture), and label all non-zero formal charges. For your reaction, also be
certain to draw the starting material, the reactant(s), and all possible products. Please label these as
"major" and "minor" (and "very minor," if needed). You may use a label more than once, but each
structure should only have one label.
Reaction J
OH
H,SO4, A
Reaction K
1. PB13,
2. NaCN
OH
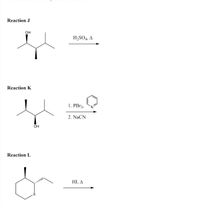
Transcribed Image Text:Reaction J
он
H,SO4, A
Reaction K
1. PB13,
2. NaCN
OH
Reaction L
HI, A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student prepares a 3.4M aqueous solution of propionic acid (C₂H₂CO₂H). Calculate the fraction of propionic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. % x10 X 5arrow_forwardTo ensure quality solutions, follow these steps: ✔️Submit correct and complete solutions. ✔️Provide step-by-step detailed explanations. ✔️Organize your solution in a clear and structured manner. ✔️Highlight key points and important steps. ✔️Address common points of confusion. By adhering to these guidelines, You can deliver high-quality solutions that are accurate, comprehensive, and easy to understand.arrow_forward7. You can see an MSDS below. Please answer the following questions related to the MSDS. a) What is the name of this chemical? b) What should you do if someone drinks the chemical? c) Would this chemical catch on fire if it was exposed to flames? d) If this chemical gets in your eye what should you do? e) What color is this chemical? f) What should you do if someone spills a small amount of the chemical?arrow_forward
- If 2.0 × 10-4 moles of H2O2H2O2 in 50 mL solution is consumed in 3 minutes and 25 seconds, what is the rate of consumption of H2O2H2O2? Express the rate in mol per liter·second to two significant digits.arrow_forwardhelp mearrow_forwardAre there any DIFFERENT, new actions you can commit to? POLLUTE OUR WATER LESThink of simple things you can do on a regular basis. It is not helpful if you cannot do it regularly. Small steps are important too. Any action you take matters.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY