
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Need help!
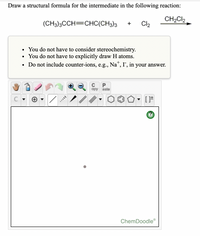
Transcribed Image Text:Draw a structural formula for the intermediate in the following reaction:
CH2Cl2
(CH3)3CCH=CHC(CH3)3
Cl2
+
• You do not have to consider stereochemistry.
• You do not have to explicitly draw H atoms.
• Do not include counter-ions, e.g., Na", I', in your answer.
opy
aste
[*
ChemDoodle®

Transcribed Image Text:Draw a structural formula for the major organic product of the following reaction:
CH2Cl2
CH3CH=CHCH2CH3
Br2
+
Show product stereochemistry IF the reactant alkene has both carbons of the double bond within a ring.
Do not show stereochemistry in other cases.
If enantiomers are formed, just draw one.
opy
aste
ChemDoodle®
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Flocculation is best described as: O A way to kill pathogens in drinking water O A way to remove dissolved chemicals from water O A way to cause suspended particles to clump and sink in water O A way of filtering large particles out of drinking waterarrow_forwardList 4 things to be considered when determining the type of irrigation system to use. a. b. c. d.arrow_forward‘’Concentrated’’ sulfuric acid solution is 95% sulfuric acid by mass (and the remainder water) and has a density of 1.64 gr/mL. ( Molar mass of H2SO4= 98 gr/mol ) What is the molarity of concentrated sulfuric acid solution? a. 18 M b. 12 M c. 16M d. 9Marrow_forward
- help mearrow_forwardA doctor has ordered for a week 0.35 grams of Carvedilol for a patient with high blood pressure. If your stock on hand consists of 25 mg tablets, how many tablets will you need for 1 day's treatment for the patient? QUESTION 13 O a. 2 O b. 14 O C. 7 O d. 20 O e. 1 QUESTION 14arrow_forwardHow does magnesium hydroxide provide relief for indigestion? Pick from the following • Magnesium hydroxide is an acid that digests food in the stomach. • Magnesium hydroxide produces magnesium chloride salt, which neutralizes HCI. • Magnesium hydroxide reacts with HCI to produce a solution that is neutral. • Magnesium hydroxide is an acid that neutralizes HCI in the stomach.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY