
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Draw a resonance structure, complete with all formal charges and lone (unshared) electron pairs, that shows the resonance interaction of the oxide with the para position in phenoxide ion. (please show which structure I need to put in the picture as my answer).
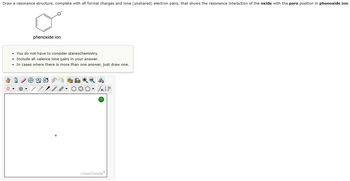
Transcribed Image Text:Draw a resonance structure, complete with all formal charges and lone (unshared) electron pairs, that shows the resonance interaction of the oxide with the para position in phenoxide ion.
O
phenoxide ion
• You do not have to consider stereochemistry.
• Include all valence lone pairs in your answer.
• In cases where there is more than one answer, just draw one.
ag
Sn [1
ChemDoodleⓇ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3) Draw two more resonance structures of the following compound. Order the structures by increasing stability.arrow_forwardDraw a valid Lewis dot structure and determine the VSEPR molecular geometry for each central atom for each of the following. When appropriate, draw all applicable resonance structures. For species in which formal charges are not all zero, determine the nonzero formal charges on the relevant atoms.arrow_forwardDraw a valid Lewis dot structure and determine the VSEPR molecular geometry for each central atom for each of the following. When appropriate, draw all applicable resonance structures. For species in which formal charges are not all zero, determine the nonzero formal charges on the relevant atoms. Thanksarrow_forward
- Draw three additional resonance structures for the anion shown below.arrow_forwardGive correct detailed Solution with explanation needed of both parts..don't give Handwritten answerarrow_forwardPlease help me the the lewis and resoance structures. Draw them out please!! A bond between CO and M, where M is a lewis acid, which forms M-CO. Where M does not have no lone pairs, has one lone pair and two lone pairs.arrow_forward
- In the following structures, write if necessary the formal charge that it must have. If you shouldn't have a load, please note that you don't have a load.arrow_forwardHelp me pls ASAParrow_forwardDraw a valid Lewis dot structure and determine the VSEPR molecular geometry for each central atom for each of the following. When appropriate, draw all applicable resonance structures. For species in which formal charges are not all zero, determine the nonzero formal charges on the relevant atoms.arrow_forward
- Please answer will rate. And explain if you desirearrow_forwardPlease don't provide handwritten solution ...arrow_forwardDraw electron dot and cross diagrams for the following compounds. Only the outermost shell of electrons needs to be shown. Use a different dot or cross for each atom or ion. (a) KH, potassium hydride (b) O3, ozone (c) Na2O, sodium oxide Provide everything stated in the instructions.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY