
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Draw a Born–Haber cycle for the formation of LiF(s) from its elements.
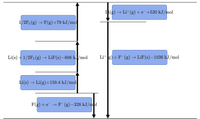
Transcribed Image Text:Li(g) → Li+ (g)+e¯ +520 kJ/mol
1/2F2 (g) → F(g)+79 kJ/mol
Li(s) +1/2F2 (g)
- LiF(s)–606 kJ/mol
Lit g) +F (g) → LiF(s)–1036 kJ/mol
Li(s) → Li(g)+159.4 kJ/mol
F(g) +e → F (g)–328 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the element Work (Wr) with an atomic number of 33 and an atomic mass of 81.03 g/mol. The following information was collected for this element Molecule/ Ion Names Wr-2 Workide WrO3-2 Workate WrO2-2 Workite WrO-3 hydroworkite Draw the Lewis structure of the molecule Workous acid and determine its electron geometry and molecular geometry. What is the mass % of work in Calcium hydroworkite.arrow_forwardWhat is noble gas electron configuration of Zn2+ ?arrow_forwardThe energy for the following reaction was measured to be -653.0 kJ/mol. K(g) + Cl(g) → KCl(s). Using this fact and data in the table below, calculate the enthalpy (in kJ/mol) required to separate the ions from the lattice for this reaction: KCl(s) → K⁺(g) + Cl⁻(g).arrow_forward
- The structure of sodium hyaluronate, a sodium salt of hyaluronic acid used in skincare products for its hydrating properties, is shown below. What is the shape of the bonds at (ii) ? Hint: atom (iii) has been completed for you as an example. (iii) bond angle: 109.5°, geometry of the electron pairs: tetrahedral, shape of the bonds: tetrahedral ОН, OH ·•·•·||||| N O H H Ol.. OH |||| iv + Naarrow_forward6. What type of bonding is Potassium likely to participate in? Why?arrow_forwardHow many electrons does a halogen atom need to com-plete its octet? Give examples of the ways a Cl atom can do so.arrow_forward
- Many nonmetallic elements have lower melting and boiling points than metallic elements because their atoms or molecules are only attracted to each other by: ionic bonds metallic bonds networks of covalent bonding intermolecular forcesarrow_forwardChoose the correct equation below for the reaction that occurs when Na2S dissociates in water.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY