
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
asap with explanation plz
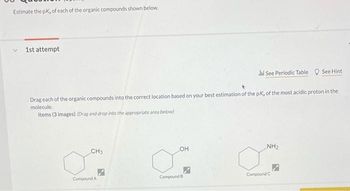
Transcribed Image Text:Estimate the pk, of each of the organic compounds shown below.
1st attempt
Drag each of the organic compounds into the correct location based on your best estimation of the pK, of the most acidic proton in the
molecule.
Items (3 images) (Drag and drop into the appropriate area below)
CH3
Compound A
OH
Compound B
Jil See Periodic Table See Hint
7
NH₂
Compound C

Transcribed Image Text:pKa ranges
3-5
Drag and drop here
8-11
Drag and drop here
16-19
Drag and drop here
35-40
Drag and drop here
45-50
Drag and drop here:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Discuss the reasons for the differences in glass transition temperature for the following pairs of polymers. Hint: check factors that affect Tg Poly(ethyl acrylate) (-24°C) and Poly(methyl methacrylate) (105°C)arrow_forwardKS-Alasia C VHL Central | Code Activation www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBjuJnuCDtT6kRBabGFF3MOAKZ_UVXON202gjJc4uFal3TAwDxMOmyojdTU6qJxvkU7ITf2iPul_KN7SCzqARI1 CkR/p8T2?10Bw7QYjlba.... Fuqua - Learn X F 12PM SPAN 101 Instructions to X O CHEMICAL REACTIONS Using molarity to find solute mass and solution volume Calculate the volume in milliliters of a 2.4M zinc nitrate solution that contains 200. g of zinc nitrate of significant digits. mL Explanation Check O X10 S + 6 0 0/5 e (Zn(NO3)₂). Be sure your answer has the correct number Saus Alasia © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility Jan 18 F Ola 3:28 Oarrow_forwardChoose a structure that best fits all of the data. 8 (ppm) multiplicity integration 2.4 3.5 quintet triplet A) Br₂C(CH3)2 B) BrCH₂CH₂CH₂Br C) BrCH₂CH(Br)CH3 D) CH3C(Br)2CH3 2H 2Harrow_forward
- Assistive Technologies for S X O MYCSU - Columbus State UX Link to AL https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-JgXZp57itWHhRgilODc5MqvhZbKYx2-U-038xjApUucKTA610SL O MEASUREMENT AND MATTER Predicting the formula of ionic compounds with commo.. Write the empirical formula for at least four ionic compounds that could be formed from the following ions: CH,CO,, NH", Fe*, Cro IIarrow_forwardKindly answer the questions and show steps in detail. I would appreciate if you could do d) as well. I only have that one question to ask and don't want to use a second question from Bartley if possible. Thank you!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY