
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Solve it
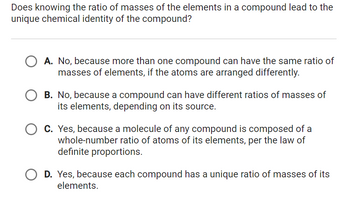
Transcribed Image Text:Does knowing the ratio of masses of the elements in a compound lead to the
unique chemical identity of the compound?
O A. No, because more than one compound can have the same ratio of
masses of elements, if the atoms are arranged differently.
OB. No, because a compound can have different ratios of masses of
its elements, depending on its source.
C. Yes, because a molecule of any compound is composed of a
whole-number ratio of atoms of its elements, per the law of
definite proportions.
D. Yes, because each compound has a unique ratio of masses of its
elements.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The answer is incorrect can you solve again?arrow_forwardStep 2: Identify the dimensions of the quantities involved The second step is to identify the dimensions of the quantities involved in the problem. For example, if the problem involves distance, time, and velocity, the dimensions of these quantities would be length, time, and length/time, respectively. Step 3: Check if the units cancel out The third step is to check if the units cancel out. To do this, multiply the quantities together and check if the units cancel out, leaving only the desired unit. For example, if you are trying to find the velocity of an object and you know its distance and time, you can multiply distance by time to get velocity. If the units cancel out, you have a physically meaningful result. Step 4: Check if the result makes sense, to do this compare the units of the result with what you would expect based on the Robles statement. Using the step hints above, answer the question. You do not have to solve. Just imagine that you're teaching a friend how…arrow_forwardPlease answer question number 1 correctly with the correct answer. there is a picture of an example of a ''Dimensional analysis Format' below. please write out all work showing the math in dimensional analysis format. Question 2: What is the molar mass of each of the following compounds? A. Phosphorus Pentachloride (PCl5) B. Uranium Hexafluoride (UF6)arrow_forward
- If given a value that is plot along x-axis: 1. Find given value along x axis. 2. From this point, trace a straight line vertically (parallel to the y-axis) until it intersects with the line graph. 3. Then, trace a line horizontally (parallel to the x axis) from the intersect to the y-axis. 4. The value of y corresponding to the given x value is where the traced line intercepts with the y- axis. Based upon what you see in the graph listed below, estimate the cost of the fence installation. 450 350 A 300 4 250 200 150 100 50 10 151 20 25 30 35 Cost (in $)arrow_forwardAm I wrong? Or is both answers correct?arrow_forwardPlease answer question number 1 correctly with the correct answer. there is a picture of an example of a ''Dimensional analysis Format' below. please write out all work showing the math in dimensional analysis format. Question 3: Calculate the molar mass of each of the following ionic Compounds: A. KMnO4 B. Ca3(PO4)2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY