
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:TRITT
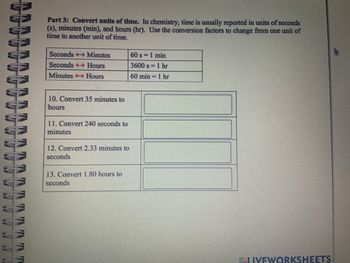
Part 3: Convert units of time. In chemistry, time is usually reported in units of seconds
(s), minutes (min), and hours (hr). Use the conversion factors to change from one unit of
time to another unit of time.
Seconds → Minutes
Seconds Hours
Minutes →→→ Hours
10. Convert 35 minutes to
hours
11. Convert 240 seconds to
minutes
12. Convert 2.33 minutes to
seconds
13. Convert 1.80 hours to
seconds
60 s = 1 min
3600 s = 1 hr
60 min 1 hr
LIVEWORKSHEETS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- PART A: an unknown mineral sample has a mass of 23.4 grams and a volume of 3 cm^3. According to the mineral density chart, what mineral is this? copper iron galena halite PART B: An unknown mineral sample has a mass of 235.32 grams and a volume of 88.8 cm^3. According to the mineral density chart, what mineral is this? galena copper aluminum quartzarrow_forwardplease make sure the answer has the correct number of significant digitsarrow_forwardNeed help with b and carrow_forward
- Bud N. Chemist must determine the density of a mineral sample. His four trials yield densities of 5.27 g/cm3, 5.28 g/cm3, 5.27 g/cm3, and 5.29 g/cm3. Bud's calculated average density was 5.28 g/cm3. Independent studies found the correct density to be 4.75 g/cm3. Which of the following statements represents the best analysis of the data? Bud's results have much greater accuracy than precision Bud's results have low accuracy and low precision Bud's results have high accuracy and high precision Bud's results have much greater precision than accuracyarrow_forwardA Part A Planet X rotates in the same manner as the earth, around an axis through its north and south poles, and is perfectly spherical. An astronaut who weighs 943.0 N on the earth weighs 910.0 N at the north pole of Planet X and only 850.0 N at its equator. The distance from the north pole to the equator is 18,850 km, measured along the surface of Planet X. How long is the day on Planet X? Express your answer in hours. ΜΕ ΑΣΦ t = Submit Request Answer Part B ? h If a 45,000 kg satellite is placed in a circular orbit 4000 km above the surface of Planet X, what will be its orbital period? Express your answer in seconds. VAZO ΜΕ ΑΣΦ ? T = Submit Request Answer Sarrow_forward1. In the olden days, US minted pennies were pure copper, but copper got so expensive that a pure copper penny was worth more than I cent. Modern pennies are composed of zinc and coated with copper. A student determines the mass of a penny to be 2.482 g. After making several scratches in a penny, the student puts the penny into hydrochloric acid, where the zinc (but not the copper) reacts with hydrochloric acid via a single replacement reaction. The hydrogen is collected at 25°C over water. The collected gas occupies 0.899 L at a total pressure of 791 mm Hg. A. Calculate the moles of hydrogen produced in the reaction. (Hint: Use the Table 1. to determine the vapor pressure of water and assume that all of the zinc in the penny dissolves.) B. Write a balanced chemical reaction for zinc and hydrochloric acid. C. Use stoichiometry to calculate the mass of zinc in the penny. D. Determine the percent zinc (by mass) in the penny.arrow_forward
- how do i set up the matharrow_forwardHow many significant figures are in 3.05481727x10^4?arrow_forward1 in = 2.54 cm, exactly 60 min = 1 hr, exactly 12 in = 1 ft, exactly 60 s = 1 min, exactly 4 qt = 1 gal, exactly 1 gal = 3.7854 L 1 BTU = 1055 J 1 lb = 453.6 g 1 mile = 1.609 km The U.S. quarter (25 cents) has a mass of 5.67 g and is about 1.55 mm thick. How many quarters are in a stack that is 1.75 ft high? Put your answer in the area provided, followed by "see upload." Conversion factors that you might find helpful are shown. Other conversion factors such as metric multipliers are not shown, as you are required to know them.arrow_forward
- 0.161 STARTING AMOUNT 0.161 km km + 10€ SHSSFE生 0.1 A city block is 0.161 km long. How many cm is this? 0.0161 0.000161 16100 161 0.001 0.00161 km 10 m 0.01 1 cm 1610 10€ 0.161 1000 100 16.1arrow_forwardYou are on a road trip. Your car travels at an average speed of 65 miles/hr, with an average gas use of 17.8 km/L. 1 liter = 1,000 mL = 0.264 gallons. 1 km = 0.621 miles. 1) If your trip takes 2.6 hours, determine how many total liters and gallons of gasoline are used on this trip. 2) How many mL (milliliters) of gasoline are used by the car each minute during this trip? 3) The density of the gasoline used on your trip is 0.755 g/mL. What is the total mass in grams, and in ounces of the gasoline used on your trip?arrow_forwardIdentify the physical change from the list below: 1) Grinding coffee 2) Baking cake 3) Converting water to hydrogen and oxygen 4) Digesting a cheeseburger 5) Burning coalarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY